Abstract
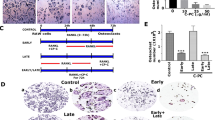
Alveolar bone loss associated with periodontal diseases is the result of osteoclastogenesis induced by bacterial pathogens. The mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP-1) is a critical negative regulator of immune response as a key phosphatase capable of dephosphorylating activated MAPKs. In this study, rat macrophages transduced with recombinant adenovirus (Ad.)MKP-1 specifically dephosphorylated activated MAPKs induced by lipopolysaccharide (LPS) compared with control cells. Bone marrow macrophages from MKP-1 knockout (KO) mice exhibited higher interleukin (IL)-6, IL-10, tumor necrosis factor (TNF)-α, and select chemokine compared with wild-type (WT) mice when stimulated by LPS. In addition, bone marrow cultures from MKP-1 KO mice exhibited significantly more osteoclastogenesis induced by LPS than when compared with WT mice. Importantly, MKP-1 gene transfer in bone marrow cells of MKP-1 KO mice significantly decreased IL-6, IL-10, TNF-α and chemokine levels, and formed fewer osteoclasts induced by LPS than compared with control group of cells. Furthermore, MKP-1 gene transfer in an experimental periodontal disease model attenuated bone resorption induced by LPS. Histological analysis confirmed that periodontal tissues transduced with Ad. MKP-1 exhibited less infiltrated inflammatory cells, less osteoclasts and less IL-6 than compared with rats of control groups. These studies indicate that MKP-1 is a key therapeutic target to control of inflammation-induced bone loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Brown LJ, Loe H . Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000 1993; 2: 57–71.
Pilot T, Miyazaki H . Periodontal conditions in Europe. J Clin Periodontol 1991; 18: 353–357.
Hall TJ, Chambers TJ . Molecular aspects of osteoclast function. Inflamm Res 1996; 45: 1–9.
Reddy SV . Regulatory mechanisms operative in osteoclasts. Crit Rev Eukaryot Gene Expr 2004; 14: 255–270.
Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B . Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun 1996; 64: 2371–2380.
Taubman MA, Valverde P, Han X, Kawai T . Immune response: the key to bone resorption in periodontal disease. J Periodontol 2005; 76: 2033–2041.
Mundy GR . Inflammatory mediators and the destruction of bone. J Periodontal Res 1991; 26: 213–217.
Hanazawa S, Amano S, Nakada K, Ohmori Y, Miyoshi T, Hirose K et al. Biological characterization of interleukin-1-like cytokine produced by cultured bone cells from newborn mouse calvaria. Calcif Tissue Int 1987; 41: 31–37.
Zou W, Bar-Shavit Z . Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res 2002; 17: 1211–1218.
Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol 1990; 145: 3297–3303.
Yang SH, Sharrocks AD, Whitmarsh AJ . Transcriptional regulation by the MAP kinase signaling cascades. Gene 2003; 320: 3–21.
Whitmarsh AJ . Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta 2007; 1773: 1285–1298.
Keyse SM . Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 2000; 12: 186–192.
Orcel P, Feuga M, Bielakoff J, De Vernejoul MC . Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol 1993; 264: E391–E397.
Nishida E, Hara Y, Kaneko T, Ikeda Y, Ukai T, Kato I . Bone resorption and local interleukin-1alpha and interleukin-1beta synthesis induced by Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res 2001; 36: 1–8.
Jiang Y, Mehta CK, Hsu TY, Alsulaimani FF . Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect Immun 2002; 70: 3143–3148.
Zhuang L, Jung JY, Wang EW, Houlihan P, Ramos L, Pashia M et al. Pseudomonas aeruginosa lipopolysaccharide induces osteoclastogenesis through a toll-like receptor 4 mediated pathway in vitro and in vivo. Laryngoscope 2007; 117: 841–847.
Ozaki Y, Ukai T, Yamaguchi M, Yokoyama M, Haro ER, Kaneko T et al. Locally administered T cells from mice immunized with lipopolysaccharide (LPS) accelerate LPS-induced bone resorption. Bone 2009; 44: 1169–1176.
Owens DM, Keyse SM . Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 2007; 26: 3203–3213.
Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 2006; 203: 131–140.
Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 2006; 103: 2274–2279.
Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T . Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol 2006; 176: 1899–1907.
Sartori R, Li F, Kirkwood KL . MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res 2009; 88: 1125–1130.
Rogers JE, Li F, Coatney DD, Otremba J, Kriegl JM, Protter TA et al. A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol 2007; 78: 1992–1998.
Wang X, Liu Y . Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal 2007; 19: 1372–1382.
Patil CS, Liu M, Zhao W, Coatney DD, Li F, VanTubergen EA et al. Targeting mRNA stability arrests inflammatory bone loss. Mol Ther 2008; 16: 1657–1664.
Kirkwood KL, Li F, Rogers JE, Otremba J, Coatney DD, Kreider JM et al. A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther 2007; 320: 56–63.
Marie PJ . Human endosteal osteoblastic cells: relationship with bone formation. Calcif Tissue Int 1995; 56 (Suppl 1): S13–S16.
Miyamoto T, Suda T . Differentiation and function of osteoclasts. Keio J Med 2003; 52: 1–7.
Yavropoulou MP, Yovos JG . Osteoclastogenesis—current knowledge and future perspectives. J Musculoskelet Neuronal Interact 2008; 8: 204–216.
Teitelbaum SL . Osteoclasts: what do they do and how do they do it? Am J Pathol 2007; 170: 427–435.
Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y . Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 2002; 169: 6408–6416.
Kim MS, Magno CL, Day CJ, Morrison NA . Induction of chemokines and chemokine receptors CCR2b and CCR4 in authentic human osteoclasts differentiated with RANKL and osteoclast like cells differentiated by MCP-1 and RANTES. J Cell Biochem 2006; 97: 512–518.
Carlson J, Cui W, Zhang Q, Xu X, Mercan F, Bennett AM et al. Role of MKP-1 in osteoclasts and bone homeostasis. Am J Pathol 2009; 175: 1564–1573.
Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T et al. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 2002; 143: 3105–3113.
Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS et al. Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum 2006; 54: 463–472.
Wilson ME, Hamilton RG . Immunoglobulin G subclass response of localized juvenile periodontitis patients to Actinobacillus actinomycetemcomitans Y4 lipopolysaccharide. Infect Immun 1992; 60: 1806–1812.
Acknowledgements
We acknowledge the continued support of the University of Michigan Orthopedic Research Labs and the Muscloskeletal Core for the microcomputed tomography. This study is supported by NIH 1R01DE018290 and 2P20 RR017696. This study was conducted in a facility constructed with support from the NIH C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yu, H., Li, Q., Herbert, B. et al. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther 18, 344–353 (2011). https://doi.org/10.1038/gt.2010.139
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.139
Keywords
This article is cited by
-
Protein tyrosine phosphatases in skeletal development and diseases
Bone Research (2022)
-
Periodontitis and diabetes interrelationships in rats: biochemical and histopathological variables
Journal of Diabetes & Metabolic Disorders (2019)
-
Dual action of highbush blueberry proanthocyanidins on Aggregatibacter actinomycetemcomitans and the host inflammatory response
BMC Complementary and Alternative Medicine (2018)
-
FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans
Lipids in Health and Disease (2015)