Abstract
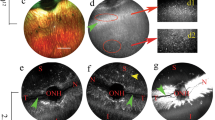
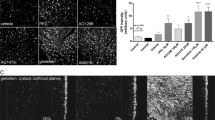
Recombinant adeno-associated viruses are important vectors for retinal gene delivery. Currently utilized vectors have relatively slow onset, and for efficient transduction it is necessary to deliver treatment subretinally, with the potential for damage to the retina. Amino-acid substitutions in the viral capsid improve efficiency in rodent eyes by evading host responses. As dogs are important large animal models for human retinitis pigmentosa, we evaluated the speed and efficiency of retinal transduction using capsid-mutant vectors injected both subretinally and intravitreally. We evaluated AAV serotypes 2 and 8 with amino-acid substitutions of surface-exposed capsid tyrosine residues. The chicken beta-actin promoter was used to drive green fluorescent protein expression. Twelve normal adult beagles were injected; four dogs received intravitreal injections and eight dogs received subretinal injections. Capsid-mutant viruses tested included AAV2(quad Y-F) (intravitreal and subretinal) and self-complementary scAAV8(Y733F) (subretinal only). Contralateral control eyes received injections of scAAV5 (subretinal) or scAAV2 (intravitreal). Subretinally delivered vectors had a faster expression onset than intravitreally delivered vectors. Subretinally delivered scAAV8(Y733F) had a faster onset of expression than scAAV5. All subretinally injected vector types transduced the outer retina with high efficiency and the inner retina with moderate efficiency. Intravitreally delivered AAV2(quad Y-F) had a marginally higher efficiency of transduction of both outer retinal and inner retinal cells than scAAV2. Because of their rapid expression onset and efficient transduction, subretinally delivered capsid-mutant AAV8 vectors may increase the efficacy of gene therapy treatment for rapid photoreceptor degenerative diseases. With further refinement, capsid-mutant AAV2 vectors show promise for retinal gene delivery from an intravitreal approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 2012; 130: 9–24.
Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010; 18: 643–650.
Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther 2009; 20: 999–1004.
Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008; 19: 979–990.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008; 358: 2231–2239.
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008; 358: 2240–2248.
Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001; 28: 92–95.
Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 2005; 12: 1072–1082.
Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Therapy 2007; 14: 292–303.
Anasagasti A, Irigoyen C, Barandika O, Lopez de Munain A, Ruiz-Ederra J . Current mutation discovery approaches in Retinitis Pigmentosa. Vision Res 2012; 75: 117–129.
Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 2010; 19: 2581–2593.
Mowat FM, Bartoe JT, Bruewer A, Dinculescu A, Boye SL, Hauswirth WW et al. Evaluation of rod photoreceptor function and preservation following retinal gene therapy in the PDE6A mutant dog. ARVO Meeting Abstracts 2012; 53: 1928.
Petit L, Lheriteau E, Weber M, Le Meur G, Deschamps JY, Provost N et al. Restoration of vision in the pde6beta-deficient dog, a large animal model of rod-cone dystrophy. Mol Ther 2012; 20: 2019–2030.
Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA 2012; 109: 2132–2137.
Pennesi ME, Stover NB, Stone EM, Chiang PW, Weleber RG . Residual electroretinograms in young Leber congenital amaurosis patients with mutations of AIPL1. Invest Ophthalmol Vis Sci 2011; 52: 8166–8173.
Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Swider M et al. Human retinal disease from AIPL1 gene mutations: foveal cone loss with minimal macular photoreceptors and rod function remaining. Invest Ophthalmol Vis Sci 2011; 52: 70–79.
Testa F, Surace EM, Rossi S, Marrocco E, Gargiulo A, Di Iorio V et al. Evaluation of Italian patients with leber congenital amaurosis due to AIPL1 mutations highlights the potential applicability of gene therapy. Invest Ophthalmol Vis Sci 2011; 52: 5618–5624.
Petersen-Jones SM, Entz DD, Sargan DR . cGMP phosphodiesterase-alpha mutation causes progressive retinal atrophy in the Cardigan Welsh corgi dog. Invest Ophthalmol Vis Sci 1999; 40: 1637–1644.
Tuntivanich N, Pittler SJ, Fischer AJ, Omar G, Kiupel M, Weber A et al. Characterization of a canine model of autosomal recessive retinitis pigmentosa due to a PDE6A mutation. Invest Ophthalmol Vis Sci 2009; 50: 801–813.
Sarra GM, Stephens C, Schlichtenbrede FC, Bainbridge JW, Thrasher AJ, Luthert PJ et al. Kinetics of transgene expression in mouse retina following sub-retinal injection of recombinant adeno-associated virus. Vision Res 2002; 42: 541–549.
Petersen-Jones SM, Bartoe JT, Fischer AJ, Scott M, Boye SL, Chiodo V et al. AAV retinal transduction in a large animal model species: comparison of a self-complementary AAV2/5 with a single-stranded AAV2/5 vector. Mol Vis 2009; 15: 1835–1842.
Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker SE, Smith AJ et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Therapy 2008; 15: 463–467.
Cook B, Lewis GP, Fisher SK, Adler R . Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 1995; 36: 990–996.
Fisher SK, Lewis GP . Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res 2003; 43: 887–897.
Fisher SK, Stone J, Rex TS, Linberg KA, Lewis GP . Experimental retinal detachment: a paradigm for understanding the effects of induced photoreceptor degeneration. Prog Brain Res 2001; 131: 679–698.
Igarashi T, Miyake K, Asakawa N, Miyake N, Shimada T, Takahashi H . Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr Eye Res 2013; 38: 569–577.
Englander M, Chen TC, Paschalis EI, Miller JW, Kim IK . Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br J Ophthalmol 2013; 97: 460–465.
Stieger K, Schroeder J, Provost N, Mendes-Madeira A, Belbellaa B, Le Meur G et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther 2009; 17: 516–523.
Auricchio A, Pseudotyped AAV . vectors for constitutive and regulated gene expression in the eye. Vision Res 2003; 43: 913–918.
Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM, Novel AAV . serotypes for improved ocular gene transfer. J Gene Med 2008; 10: 375–382.
Boye SE, Boye SL, Lewin AS, Hauswirth WW . A comprehensive review of retinal gene therapy. Mol Ther 2013; 21: 509–519.
Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther 2007; 15: 1323–1330.
Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008; 105: 7827–7832.
Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 2009; 17: 463–471.
Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther 2011; 19: 293–301.
Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther 2011; 19: 234–242.
Ku CA, Chiodo VA, Boye SL, Goldberg AF, Li T, Hauswirth WW et al. Gene therapy using self-complementary Y733F capsid mutant AAV2/8 restores vision in a model of early onset Leber congenital amaurosis. Hum Mol Genet 2011; 20: 4569–4581.
Boye SL, Conlon T, Erger K, Ryals R, Neeley A, Cossette T et al. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest Ophthalmol Vis Sci 2011; 52: 7098–7108.
Dinculescu A, Estreicher J, Zenteno JC, Aleman TS, Schwartz SB, Huang WC et al. Gene therapy for retinitis pigmentosa caused by MFRP mutations: human phenotype and preliminary proof of concept. Hum Gene Ther 2012; 23: 367–376.
Deng WT, Dinculescu A, Li Q, Boye SL, Li J, Gorbatyuk MS et al. Tyrosine-mutant AAV8 delivery of human MERTK provides long-term retinal preservation in RCS rats. Invest Ophthalmol Vis Sci 2012; 53: 1895–1904.
Martino AT, Basner-Tschakarjan E, Markusic DM, Finn JD, Hinderer C, Zhou S et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013; 121: 2224–2233.
Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One 2013; 8: e59142.
Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA 2013; 110: E517–E525.
Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J et al. Targeting Photoreceptors via Intravitreal Delivery Using Novel, Capsid-Mutated AAV Vectors. PLoS One 2013; 8: e62097.
Garcia-Sanchez GA, Gil-Carrasco F, Roman JJ, Brooks DE, Alvarez-Clau A, Hosgood G et al. Measurement of retinal nerve fiber layer thickness in normal and glaucomatous Cocker Spaniels by scanning laser polarimetry. Vet Ophthalmol 2007; 10 (Suppl 1): 78–87.
Hernandez-Merino E, Kecova H, Jacobson SJ, Hamouche KN, Nzokwe RN, Grozdanic SD . Spectral domain optical coherence tomography (SD-OCT) assessment of the healthy female canine retina and optic nerve. Vet Ophthalmol 2011; 14: 400–405.
Ferguson LR, Dominguez IiJM, Balaiya S, Grover S, Chalam KV . Retinal Thickness Normative Data in Wild-Type Mice Using Customized Miniature SD-OCT. PLoS One 2013; 8: e67265.
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 2013; 5: 189ra76.
Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 2009; 17: 2096–2102.
Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, Schaffer DV et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther 2010; 21: 571–578.
Heegaard S . Structure of the human vitreoretinal border region. Ophthalmologica 1994; 208: 82–91.
Matsumoto B, Blanks JC, Ryan SJ . Topographic variations in the rabbit and primate internal limiting membrane. Invest Ophthalmol Vis Sci 1984; 25: 71–82.
Wolter JR . Pores in the Internal Limiting Membrane of the Human Retina. Acta Ophthalmol 1964; 42: 971–974.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006; 50: 23–33.
Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S et al. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci 2007; 48: 3821–3826.
Lin B, Koizumi A, Tanaka N, Panda S, Masland RH . Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA 2008; 105: 16009–16014.
Zhang Y, Ivanova E, Bi A, Pan ZH . Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci 2009; 29: 9186–9196.
Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther 2011; 19: 1220–1229.
Bruewer AR, Mowat FM, Bartoe JT, Boye SL, Hauswirth WW, Petersen-Jones SM . Evaluation of lateral spread of transgene expression following subretinal AAV-mediated gene delivery in dogs. PLoS One 2013; 8: e60218.
Gearhart PM, Gearhart C, Thompson DA, Petersen-Jones SM . Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol 2010; 128: 1442–1448.
Mowat FM, Bartoe JT, Bruewer A, Smith AJ, Bainbridge JB, Ali RR et al. RPE65 gene therapy promotes survival of S-cones in the RPE65-deficient dog. Invest Ophthalmol Vis Sci 2011; 52: 1.
Li A, Zhu X, Brown B, Craft CM . Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci 2003; 44: 996–1007.
Acknowledgements
We thank Janice Querubin and Lisa Allen for animal assistance, Joe Hauptman for statistical assistance, Cheryl Craft for hCAR antibody and Vince Chiodo for AAV purification. JTB and FMM acknowledge the following funding sources: Michigan State University College of Veterinary Medicine Endowed Research Fund. SPJ acknowledges the following funding sources: The Glassen Memorial Foundation, Myers-Dunlap Endowment for Canine Health. WWH acknowledges the following funding sources: NIH grant EY021721 and grants from the Macula Vision Research Foundation, Foundation Fighting Blindness, Eldon Family Foundation, Overstreet Fund and Research to Prevent Blindness, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WWH and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. SLB and WWH hold a patent (No. 8 298 818) that covers some aspects of the AAV technology used in this study. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mowat, F., Gornik, K., Dinculescu, A. et al. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther 21, 96–105 (2014). https://doi.org/10.1038/gt.2013.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.64
Keywords
This article is cited by
-
Outer retinal transduction by AAV2-7m8 following intravitreal injection in a sheep model of CNGA3 achromatopsia
Gene Therapy (2022)
-
Natural history of retinal degeneration in ovine models of CLN5 and CLN6 neuronal ceroid lipofuscinoses
Scientific Reports (2022)
-
Virus Vectors for Optogenetic Prosthetization of the Retina
Neuroscience and Behavioral Physiology (2020)
-
Retina transduction by rAAV2 after intravitreal injection: comparison between mouse and rat
Gene Therapy (2019)
-
AAVrh-10 transduces outer retinal cells in rodents and rabbits following intravitreal administration
Gene Therapy (2019)