Abstract
Perivascular adipose tissue (PVAT) is implicated in the regulation of vascular function in the physiological state, but the modulatory effect of PVAT on vasculature during obesity is poorly understood. Endothelial nitric oxide synthase (eNOS), AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) participate in the regulation of vascular function. We therefore investigated whether PVAT has a potential role through the AMPK/mTOR pathway in obesity-related vascular dysfunction. Wistar rats on a high-fat diet (HFD) for 6 months had higher periaortic fat mass compared with rats on a chow diet (3.31±0.56 vs. 2.34±0.28 g, P<0.05). Obesity-related impairment of endothelium-dependent relaxation of the aorta was markedly attenuated by temporary periaortic fat removal whereas obesity-related enhancement of contractile performance was unaffected. Rats on an HFD had thicker aortic tunica medias (180.06±7.56 vs. 128.14±13.21 μm for rats on a chow diet, P<0.01) and larger periaortic adipocytes than rats on a chow diet (1209.00±62.65 vs. 447.20±21.31 μm2, respectively, P<0.01). Furthermore, mesenteric arterial rings incubated with periaortic fat from rats on an HFD demonstrated lower endothelium-dependent relaxation. This effect was absent in mesenteric arterial rings incubated with periaortic fat from rats on a chow diet. Moreover, an HFD led to a downregulation of AMPK and eNOS in the aorta with a concurrent upregulation of mTOR. In a parallel in vitro study, culturing vascular smooth muscle cells with periaortic adipocytes from rats on an HFD reduced the AMPK phosphorylation and increased mTOR phosphorylation, and the latter one was blocked by the incubation of compound C. We conclude that PVAT likely impacts obesity-related vascular dysfunction and remodeling through impairment of eNOS-mediated vasodilatation and the AMPK/mTOR pathway.
Similar content being viewed by others
Introduction
Obesity is a major risk factor for diabetes, metabolic syndrome, hypertension, coronary heart diseases and peripheral vascular disease. Recent studies have highlighted the role of ectopic fat depots in the pathogenesis of cardiometabolic diseases. The perivascular adipose tissue (PVAT) is a functional component of the vasculature, exerting paracrine influences on vascular reactivity and vascular proliferation.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Under physiological conditions, PVAT acts as a vasoactive fat,11 attenuating vasoconstriction in rodents and human through different mechanisms.12, 13, 14, 15, 16, 17 Conversely, perivascular fat has also been reported to enhance the contraction of the rat aortae and mesenteric arteries.18 A recent study showed that PVAT induces a proinflammatory state in high-fat diet (HFD)-induced obese mice.19 AMP-activated protein kinase (AMPK), a member of a metabolite-sensing protein kinase family, has been implicated in the regulation of vascular function through increased phosphorylation of endothelial nitric oxide synthase (eNOS).20, 21 In addition, AMPK has key roles in both vascular smooth muscle cell (VSMC) proliferation and metabolism.22 One of the major downstream signaling pathways regulated by AMPK is the mammalian target of rapamycin (mTOR) pathway.23 Rapamycin is currently used in drug-eluting stents to prevent restenosis after angioplasty by inhibiting VSMC proliferation. However, no studies have examined whether PVAT could affect the adjacent vasculature by acting on the AMPK/mTOR pathway. This study studied the effect of PVAT on vascular function, vascular remodeling and the AMPK/mTOR pathway in HFD-induced obese rats.
Methods
Chemicals
Phenylephrine (PE), acetylcholine (ACh) and nitroglycerin (NTG) were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Animals
Male Wistar rats, 6–8 weeks of age, were obtained from Charles River (Sulzfeld, Germany). Rats were housed under 12/12 h day/night conditions, and food and water were given ad libitum to all animals. The local Animal Care and Use Committee approved all animal care and use procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Animals were given the standard chow diet (control group, n=18) or an HFD (HFD group, n=18) for 6 months.
Aortic vascular reactivity assay
Aortic vascular reactivity assays were performed as described earlier.24, 25 After the animals were anesthetized with pentobarbital sodium (50 mg kg−1 body mass, i.p.), aortae were dissected and immediately placed in cold Krebs solution containing (mM): NaCl 119; NaHCO3 25; glucose 11.1; KCl 4.7; KH2PO4 1.2; MgSO4 1.2 and CaCl2 2.5. The thoracic aorta in each animal was dissected into four adjacent 3.0 mm ring sections. Two rings were dissected with periaortic adipose tissue, whereas the other two rings cleaned of all adherent tissue. These rings were then placed onto force transducer arms and submerged in tissue baths containing a Krebs solution with 95% O2/5% CO2 compressed gas. Force transducers were connected to a PowerLab signal transduction unit (AD Instruments, Sydney, Australia), and data were continuously recorded and further analyzed using the Chart 6 program (AD Instruments). Rings were stretched to 2 g over 1 h. A single dose of KCl was applied (60 mM) to determine smooth muscle contractility. The rings were then rinsed until the baseline tone returned. The contractile response to PE (10−10–10−6 M) was assessed. The relaxation to ACh(10−9–10−4 M) and NTG (10−9–10−5 M) was determined in rings contracted by 10–5 M PE.
Mesenteric vascular reactivity assay
Vascular reactivity in the isolated mesenteric artery rings was measured using an isometric myograph (Danish Myotech Technology, Aarhus, Denmark) as described.26 After the rats were anesthetized, the mesenteric bed was dissected and immediately placed in cold Krebs solution. The second branches of the mesenteric arterial bed were carefully dissected out. Arterial segments (100–200 μm in lumen diameter, ∼5 mm in length) were mounted in the Myograph System. Each ring was bathed in Krebs solution aerated with 95% O2 and 5% CO2 at 37 °C, pH 7.4. After measurement of passive-tension internal circumference characteristics, tension was set to the estimated in vivo internal circumference. After a 60 min stabilization period, the functional integrity of the rings was confirmed by contraction with KCl (60 mM). The presence of functional endothelium was confirmed by the ability of ACh (10–6 M) to produce relaxation of pre-contraction with PE (10–6 M) in the presence of propranolol (10–7 M) to block β-adrenoceptors. Segments relaxing >80% were used. Cumulative concentration–response curves to PE (10−9–10−5 M) were obtained. The mesenteric rings were then pre-contracted with PE (10−5 M). Cumulative concentration–response curves of ACh (10−9–10−4 M) and NTG (10−9–10−4 M) in the absence or presence of periaortic fat were obtained to determine the endothelium-dependent and endothelium-independent relaxations, respectively. The relaxant responses are expressed as percentage reduction in the tone induced by PE (10−5 M).
Cell culture
VSMCs were obtained from thoracic aortae of rats and cultured using the tissue explant method as previously described by our group.27 Perivascular adipocytes were obtained from periaortic fat tissue and cultured using the tissue explant method as described earlier.28 VSMC/adipocyte cocultures in the presence or absence of 10 μM compound C, an AMPK inhibitor (Calbiochem, San Diego, CA, USA), were established as previously described on the opposite sides of porous polyethylene terepthalate culture plastic inserts, which had an effective culture area of 0.3 cm2, a pore size of 0.4 μm and a pore density of 1 × 108 per cm2 (Becton Dickinson, Franklin Lakes, NJ, USA).
Western blotting
Immunoblotting of total and phosphorylated AMPK, mTOR, eNOS, phosphoinositide 3-kinase (PI3K) and protein kinase B (PKB/Akt) was performed using standard techniques as previously reported.28 VSMCs cocultured as above or aortic tissue were homogenized in high-salt buffer containing NaCl 600 mM, MOPS 40 mM, DTT 1 mM, leupeptin 1 μg ml−1, aprotinin 1 μg ml−1, phenylmethylsulfonyl fluoride 50 mM. Cells or vascular tissue were scraped off, transferred to Eppendorf tubes and sonicated for 5 s. Protein supernatants were separated by centrifugation, and protein concentrations were determined with Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were separated using 10% sodium dodecyl sulfate polyacrylamide gels and transferred to Hybond-ECL nitrocellulose membranes (NEN Life Science Products, Boston, MA, USA) at 100 V for 1 h. Membranes were blocked for 8 h at 4 °C with blocking buffer containing tromethamine hydrochloride-buffered saline and 0.1% polysorbate-20 with 5% wt/vol nonfat dry milk. Membranes were incubated with primary rabbit monoclonal IgG (1:1000) anti-AMPK, anti-p-AMPK, anti-mTOR, anti-p-mTOR, anti-eNOS, anti-p-eNOS, anti-PI3K, anti-p-PI3K, anti-Akt and anti-p-Akt (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 8 h at 4 °C. After washing, membranes were incubated with secondary antibodies (goat anti-rabbit horseradish peroxidease, 1:2000) for 1 h at room temperature and washed extensively. Each sample was processed three to six times.
Histology
Aortic tunica media thickness was determined according to established techniques.29 Aortic tissue was fixed in 10% formaldehyde/phosphate-buffered saline and embedded in paraffin. After routine histological procedures, cross-sections proximal to the first intercostal artery were stained with Victoria blue and Ponceau S and studied under × 200 magnification. Pictures were obtained with a digital camera using a Nikon TE2000 microscope (Nikon Corporation, Tokyo, Japan). The tunica media was defined as the region between the internal and external elastic laminae, which were measured at four orthogonal points of the arterial sections for three consecutive aortic rings per animal using the imaging software NIS-Elements 3.0 (Nikon Corporation).
Periaortic adipocyte size was measured as described earlier.30 Periaortic adipose tissue from six rats was harvested, fixed with 10% buffered formalin, paraffin embedded and stained with hematoxylin and eosin. Images were captured using a Nikon TE2000 microscope (Nikon Corporation), and the area for a total of 100 adipocytes drawn from each rat was determined using the imaging software NIS-Elements 3.0 (Nikon Corporation).
Statistics
Data are mean±s.e.m. The half maximal effective agonist concentration (EC50) and maximum response (Emax) were calculated from individual agonist concentration–response curves using GraphPad Prism 3.0 (San Diego, CA, USA). The statistical differences in mean values were assessed by Student's t-test or one-way analysis of variance with Bonferroni's multiple comparison post hoc tests, as appropriate. Two-sided P-values below 0.05 were considered statistically significant.
Results
Effects of PVAT on aortic reactivity
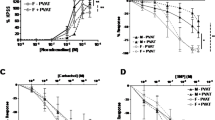
Compared with rats on a chow diet, body weight and adipose tissue mass were markedly increased in rats on an HFD for 6 months (Table 1). However, the tail-cuff systolic blood pressure was not different between two groups (127±4 mm Hg in HFD vs. 123±5 mm Hg in chow diet, P>0.05). The PE-induced contractile response of aortic rings was markedly enhanced in HFD-fed rats, especially in the presence of PVAT (Figures 1a and b) (Table 2). Furthermore, endothelium-dependent relaxation was significantly reduced in rats on an HFD compared with rats on a chow diet only in the presence of PVAT (Figures 1c and d) (Table 2). However, endothelium-independent relaxation was unaffected in the presence or absence of PVAT in rats on either a chow diet or an HFD (Figures 1e and f) (Table 2).
Effects of PVAT on aortic reactivity. PE-induced contraction (a, b) and ACh- or NTG-elicited relaxation (c–f) were assessed in freshly isolated aortic rings with or without PVAT from rats placed on the control and the high-fat diet (HFD). Results are mean±s.e.m. of 6–8 rings from 6–8 rats per group. *P<0.05 between curves.
Effect of PVAT on reactivity of mesenteric artery
To determine whether PVAT affects small blood vessels, freshly isolated mesenteric arterial rings were incubated with periaortic fat for 60 min. PE-induced constriction did not differ between arterial rings of rats on a chow diet and an HFD, regardless of the presence or absence of periaortic fat (Figures 2a and b) (Table 3). Endothelium-dependent relaxation of arterial rings was significantly reduced in rats on an HFD when co-incubated with periaortic fat compared with rings without periaortic fat incubation. This effect was absent in rats on a chow diet (Figures 2c and d) (Table 3). Endothelium-independent relaxation did not differ between the two groups (Figures 2e and f) (Table 3).
Effect of PVAT on the reactivity of the mesenteric artery. PE-induced contraction (a, b) and ACh- (c, d) or NTG-elicited relaxation (e, f) were assessed in freshly isolated mesenteric arterial rings in the presence or absence of their periaortic adipose tissue. Results are mean±s.e.m. of 5–10 rings from 5–10 rats per group; *P<0.05 between curves.
HFD reduces activity of eNOS
Next, we examined whether the impairment of vascular relaxation induced by an HFD and PVAT is associated with a change of eNOS activity in aortae. The level of phosphorylated eNOS, but not total eNOS, was markedly decreased in the aortae of rats on an HFD compared with rats on a chow diet (Figure 3).
The eNOS expression and phosphorylation in the aorta. The total eNOS and p-eNOSSer1777 in aortic tissue from chow- (control) or HFD-fed rats were detected by western blotting. Bar graphs show the relative phosphorylation normalized to the control group. Results are mean±s.e.m. of 4–8 independent experiments from 4–8 rats. **P<0.01 between the two groups.
Remodeling of aortae and PVAT in rats on an HFD
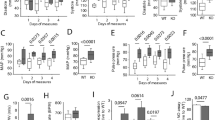
To determine whether an HFD can cause vascular and adipose tissue remodeling, the aortic tunica media in both groups was analyzed. Sections of Victoria blue- and Ponceau S-stained aortae were examined (Figure 4a). The thickness of the aortic tunica media was significantly increased in rats on an HFD compared with rats on a chow diet (180.1±7.6 vs. 128.1±13.2 μm, respectively, P<0.01) (Figure 4b). In addition, histological examination showed that large-sized adipocytes were more abundant in the PVAT of rats on an HFD compared with rats on a chow diet (Figure 4c). Figure 4d shows that the average size of periaortic adipocytes was significantly larger in rats on an HFD compared with rats on a chow diet (447.2±21.3 vs. 1209.0±62.7 μm2, respectively, P<0.01).
Remodeling of the aorta and PVAT in rats on an HFD. (a) The images show cross-sections of Victoria blue- and Ponceau S-stained aortae from each group of rats. Left panel, control group; right panel, HFD group. Scale bar indicates 100 μm; magnification, × 200. (b) The graph shows quantification of the thickness of the aortic tunica media. Results are mean±s.e.m. (n=3 rats per group). **P<0.01 vs. control. (c) Sections of periaortic fat from each group of rats (n=3 per group) were stained with H&E; left panel, control group; right panel, HFD group. Scale bar indicates 20 μm; magnification, × 400. (d) The graph shows quantification of the periaortic adipocyte size. Results are mean±s.e.m. (n=100 adipocytes). **P<0.01 vs. control group. A full color version of this figure is available at the Hypertension Research journal online.
Expression of AMPK and mTOR in aortae from rats on an HFD
Previous studies have shown that AMPK suppresses VSMC proliferation by increasing the activity of the TSC2-mTOR pathway, an important cell-cycle regulator.31 The AMPK expression and phosphorylation in the aortae from rats on an HFD were significantly decreased compared with those on a chow diet (Figure 5a). As shown in the representative bands, total and phosphorylated mTOR levels were significantly increased by HFD feeding (Figure 5b). However, the ratio of phosphorylated mTOR to total mTOR was unchanged (Figure 5b). In addition, the PI3K and Akt protein expression and phosphorylation in aorta were similar in the two groups (Figures 5c and d).
AMPK, mTOR, PI3K and Akt protein expression and phosphorylation in aortic tissue. AMPK/p-AMPKThr172 (a), mTOR/p-mTORSer2448 (b), PI3K/p-PI3K (c) and Akt/p-Akt (d) in aortic tissue from chow- (control) or HFD-fed rats were detected by western blotting. Bar graphs show the relative phosphorylation normalized to the control group. Results are mean±s.e.m. of 4–8 independent experiments from 4–8 rats. **P<0.01 between the two groups.
Effect of PVAT on AMPK/mTOR expression in cultured VSMCs
To determine whether PVAT affected the AMPK/mTOR expression in VSMCs, VSMCs were co-cultured with periaortic adipocytes in the presence or absence of the AMPK inhibitor compound C (10 μM) for 24 h. In VSMCs, periaortic adipocyte incubation significantly decreased p-AMPK and increased p-mTOR level (Figures 6a and b). The coculture-induced increase in mTOR phosphorylation was significantly attenuated by the presence of compound C, whereas the coculture-induced decrease in AMPK phosphorylation was unaffected (Figures 6a and b). Additionally, PI3K and Akt protein expression and phosphorylation were not affected by either the periaortic adipocyte incubation or the treatment of compound C (Figures 6c and d).
AMPK, mTOR, PI3K and Akt protein expression and phosphorylation in cultured vascular smooth muscle cells (VSMCs). AMPK/p-AMPKThr172 (a), mTOR/p-mTORSer2448 (b), PI3K/p-PI3K (c) and Akt/p-Akt (d) in VSMCs co-cultured with periaortic adipocytes from HFD-fed rats were detected by Western blotting. Bar graphs show the relative phosphorylation normalized to the control group. Control=no periaortic co-culture, CC=compound C. Results are mean±s.e.m. (n=3–6 independent experiments). **P<0.01 between the two groups.
Discussion
This study shows that an HFD in rats leads to decreased endothelium-dependent aortic relaxation and increased contraction, especially in the presence of PVAT. Similarly, endothelium-dependent relaxation of mesenteric arteries was significantly lower when arterial rings were incubated with PVAT from rats on an HFD. The decreased endothelium-dependent relaxation in HFD rats was accompanied with a vascular eNOS reduction in rats on an HFD. In addition, the aortic tunica media thickness and adipocyte size in the PVAT were significantly increased in rats on an HFD compared with rats on a chow diet. In vivo, rats on an HFD had lower AMPK and higher mTOR expression in aortae compared with rats on a chow diet. In vitro, a similar expression pattern was seen in VSMCs co-cultured with PVAT from rats on an HFD. These results suggest that a PVAT abnormality contributes to vascular dysfunction and remodeling by modulating the vascular AMPK/mTOR pathway in rats on an HFD.
The adventitia is composed of adipose tissue and a perivascular nerve network, but the latter does not participate in the relaxation of rat aortae.32 The PVAT can affect vascular function by virtue of its location and secretory function. Previous studies reported that PVAT has a dual role in regulating vascular function, both attenuating the response to vasoconstrictors and promoting perivascular nerve excitation.10, 17 There are several proposed mechanisms for the effect of PVAT on the vasculature, including releasing adipocyte-derived relaxing factor, opening potassium channels and promoting NO production,12, 16, 17 and the function of PVAT is modified by different physiologic and pathophysiological states.33, 34 As reported, visfatin is involved in PVAT-induced VSMC proliferation through extracellular signal-regulated kinase (ERK 1/2) and p38 signals.35 Additionally, renin-angiotensin system components, eNOS/NO and hydrogen peroxide have also been identified in PVAT.36 Classic adipokines, such as leptin and adiponectin, are also involved in the paracrine effect of PVAT.37 Given its complexity and importance, the distinct paracrine elements of PVAT warrant further studies.
Although a reduction in the anticontractile effect of perivascular fat in mesenteric arterioles has been reported in spontaneously hypertensive rats,5 few studies have examined the role of PVAT in obesity. This study shows for the first time that the presence of PVAT impairs relaxation in both the conduit artery and small arterioles in HFD-induced obese rats. Furthermore, obese rats on an HFD have larger adipocytes in their PVAT and a thicker aortic tunica media. Given these novel findings, we decided to investigate their underlying mechanisms.
AMPK is a member of a metabolite-sensing protein kinase family present in all eukaryotes and is thought to regulate cellular proliferation in response to energy status or nutrient availability. mTOR, an evolutionarily conserved serine/threonine kinase, integrates nutrient and mitogen signals to regulate cell growth and cell division through protein translation/synthesis. AMPK activation suppresses mTOR signaling and increases the activity of the TSC1–TSC2 complex.38 These findings suggest that activation of AMPK inhibits the mTOR activity to limit protein synthesis. NO has a critical role in the modulation of vascular relaxation, and the activity of the enzyme responsible for the production of NO, eNOS is regulated by a series of protein kinases, including AMPK.21 AMPK is required for adiponectin-, thrombin- and histamine-induced NOS phosphorylation and subsequent NO production in the endothelium.21
The present results show that in aortae from HFD-induced obese rats, the levels of AMPK and p-eNOS are downregulated whereas the mTOR expression is increased. Furthermore, incubating cultured VSMCs with PVAT reduces AMPK expression and increases mTOR expression. Our results support the notion that an abnormal AMPK/mTOR signaling pathway may be responsible for vascular dysfunction and remodeling in HFD-induced obese rats. Moreover, the inhibitory effect of compound C on the coculture-induced increase in mTOR phosphorylation further confirmed that the mTOR is regulated by PVAT through AMPK activation.
Obesity is one of major risk factors for coronary heart disease. Clinical studies have shown that rapamycin-coated stents prevent restenosis in both animal models and patients with coronary heart disease. Rapamycin, by interacting with mTOR, blocks cell-cycle progression from G1 to S, thus preventing T-cell proliferation and smooth muscle cell migration.39 This study shows that obesity causes an abnormality in the AMPK/mTOR pathway in blood vessels. Although the mTOR is affected by the PI3K/Akt pathway and to phosphorylate the p70S6K and 4EBP1,40 this study shows that total and phosphorylated PI3K and Akt protein levels in the aortae from rats on an HFD were similar to those in aortas from rats on chow diet. These results further indicated that AMPK/mTOR signal pathway might be essential for the regulation of obesity-related vascular dysfunction and remodeling through inhibition of endothelial NO-mediated vasodilatation. Thus, the AMPK/mTOR pathway may become another therapeutic target for the intervention of obesity-related vascular diseases, especially in the presence of abnormal PVAT.
The pathophysiological mechanism by which PVAT may mediate vascular dysfunction and remodeling has not been fully understood. Increased production of reactive oxygen species involved in the mechanisms promoting endothelial dysfunction and leading to vascular remodeling has been suggested as a potential mediator.41 It has been reported that PVAT induces a proinflammatory state in HFD-induced obese mice.19 Inflammatory cell infiltration into perivascular tissue and consequently inflammatory factors migration into the vascular wall might also be the possible mechanisms leading to vascular dysfunction and remodeling.
References
Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD . Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation 2007; 14: 389–402.
Iacobellis G, Gao YJ, Sharma AM . Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr Diab Rep 2008; 8: 20–24.
Stern N, Marcus Y . Perivascular fat: innocent bystander or active player in vascular disease? J Cardiometab Syndr 2006; 1: 115–120.
Thalmann S, Meier CA . Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res 2007; 75: 690–701.
Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS . Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 2006; 26: 1297–1302.
Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA . Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 2005; 25: 2594–2599.
Barandier C, Montani JP, Yang Z . Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol 2005; 289: H1807–H1813.
Gollasch M, Dubrovska G . Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci 2004; 25: 647–653.
Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R . Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol 2007; 58: 591–610.
Gao YJ . Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des 2007; 13: 2185–2192.
Yudkin JS, Eringa E, Stehouwer CD . ‘Vasocrine’ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 2005; 365: 1817–1820.
Dubrovska G, Verlohren S, Luft FC, Gollasch M . Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 2004; 286: H1107–H1113.
Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M . Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 2004; 44: 271–276.
Reifenberger MS, Turk JR, Newcomer SC, Booth FW, Laughlin MH . Perivascular fat alters reactivity of coronary artery: effects of diet and exercise. Med Sci Sports Exerc 2007; 39: 2125–2134.
Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, Lamy A, Semelhago L, Lee RM . Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 2005; 130: 1130–1136.
Brandes RP . The fatter the better? Perivascular adipose tissue attenuates vascular contraction through different mechanisms. Br J Pharmacol 2007; 151: 303–304.
Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM . Periadventitial fat releases a vascular relaxing factor. FASEB J 2002; 16: 1057–1063.
Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM . Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res 2006; 71: 363–373.
Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL . Proinflammatory phenotype of perivascular adipocytes influence of high-fat feeding. Circ Res 2009; 104: 541–549 online.
Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A . Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol 2007; 581 (Pt 3): 1163–1171.
Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland Jr T, Shyy JY . AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol 2006; 26: 1281–1287.
Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E . Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 2005; 97: 837–844.
Shaw RJ . LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009; 196: 65–80.
Grobe JL, Katovich MJ . Alterations in aortic vascular reactivity to angiotensin 1-7 in 17-beta-estradiol-treated female SD rats. Regul Pept 2006; 133: 62–67.
Zhu Z, Tepel M, Neusser M, Mehring N, Zidek W . Effect of captopril on vasoconstriction and Ca2+ fluxes in aortic smooth muscle. Hypertension 1993; 22: 806–811.
Matsumoto T, Miyamori K, Kobayashi T, Kamata K . Apocynin normalizes hyperreactivity to phenylephrine in mesenteric arteries from cholesterol-fed mice by improving endothelium-derived hyperpolarizing factor response. Free Radic Biol Med 2006; 41: 1289–1303.
Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, Zhu Z, Tepel M . Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension 2009; 53: 70–76.
Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M . Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res 2007; 100: 1063–1070.
Pereira LM, Bezerra DG, Mandarim-de-Lacerda CA . Aortic wall remodeling in rats with nitric oxide deficiency treated by enalapril or verapamil. Pathol Res Pract 2004; 200: 211–217.
Mori Y, Itoh Y, Tajima N . Angiotensin II receptor blockers downsize adipocytes in spontaneously type 2 diabetic rats with visceral fat obesity. Am J Hypertens 2007; 20: 431–436.
Motoshima H, Goldstein BJ, Igata M, Araki E . AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 2006; 574 (Pt 1): 63–71.
Rocha ML, Bendhack LM . Relaxation evoked by extracellular Ca(2+) in rat aorta is nerve-independent and involves sarcoplasmic reticulum and L-type Ca(2+) channel. Vascul Pharmacol 2009; 50: 98–103.
Engeli S . Is there a pathophysiological role for perivascular adipocytes? Am J Physiol Heart Circ Physiol 2005; 289: H1794–H1795.
Lee RM, Lu C, Su LY, Werstuck G, Gao YJ . Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J Hypertens 2009; 27: 118–131.
Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY . Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 2009; 81: 370–380.
Gao YJ, Lu C, Su LY, Sharma AM, Lee RM . Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 2007; 151: 323–331.
Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M . Adiponectin is a novel humoral vasodilator. Cardiovasc Res 2007; 75: 719–727.
Inoki K, Zhu T, Guan KL . TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590.
Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR . Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest 1996; 98: 2277–2283.
Ropelle ER, Pauli JR, Fernandes MF, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JA, Franchini KG, Velloso LA, Saad MJ, Carvalheira JB . A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 2008; 57: 594–605.
Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL . Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 2009; 54: 1384–1392 online.
Acknowledgements
We thank Dr Yu Huang for helpful comments on the paper. We also thank Tingbing Cao, Lijuan Wang and Xiaoli Feng for their technical assistance. This study was supported by the Natural Science Foundation of China (30670839) and the 973 program (2006CB503804).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ma, L., Ma, S., He, H. et al. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res 33, 446–453 (2010). https://doi.org/10.1038/hr.2010.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.11
Keywords
This article is cited by
-
MicroRNAs and obesity-induced endothelial dysfunction: key paradigms in molecular therapy
Cardiovascular Diabetology (2020)
-
Renal denervation improves vascular endothelial dysfunction by inducing autophagy via AMPK/mTOR signaling activation in a rat model of type 2 diabetes mellitus with insulin resistance
Acta Diabetologica (2020)
-
Beneficial effects of murtilla extract and madecassic acid on insulin sensitivity and endothelial function in a model of diet-induced obesity
Scientific Reports (2019)
-
Emerging Roles of Sympathetic Nerves and Inflammation in Perivascular Adipose Tissue
Cardiovascular Drugs and Therapy (2019)
-
Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword
Cardiovascular Diabetology (2018)