Abstract
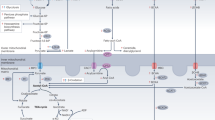
Myocardial ischemia produces an energy-deficient state in heart muscle, which if not corrected can lead to cardiomyocyte death. AMP-activated protein kinase (AMPK) is a key kinase that can increase energy production in the ischemic heart. During ischemia a rapid activation of AMPK occurs, resulting in an activation of both myocardial glucose uptake and glycolysis, as well as an increase in fatty acid oxidation. This activation of AMPK has the potential to increase energy production, thereby protecting the heart during ischemic stress. However, at clinically relevant high levels of fatty acids, ischemia-induced activation of AMPK also stimulates fatty acid oxidation during and following ischemia. This can contribute to ischemic injury secondary to an inhibition of glucose oxidation, which results in a decrease in cardiac efficiency. As a result, AMPK activation has the potential to be either beneficial or harmful in the ischemic heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hardie DG, Carling D . The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem 1997; 246: 259–273.
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD . High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 1995; 270: 17513–17520.
Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS et al. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta 1996; 1301: 67–75.
Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL et al. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett 2001; 505: 348–352.
Beauloye C, Marsin AS, Bertrand L, Vanoverschelde JL, Rider MH, Hue L . The stimulation of heart glycolysis by increased workload does not require AMP-activated protein kinase but a wortmannin-sensitive mechanism. FEBS Lett 2002; 531: 324–328.
Russell III RR, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 2004; 114: 495–503.
Baron SJ, Li J, Russell III RR, Neumann D, Miller EJ, Tuerk R et al. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res 2005; 96: 337–345.
Li J, Miller EJ, Ninomiya-Tsuji J, Russell III RR, Young LH . AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MPAK to TAB1 in the ischemic heart. Circ Res 2005; 97: 872–879.
Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 2005; 11: 1096–1103.
Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am J Physiol Endocrinol Metab 2006; 290: E780–E788.
Russell III RR, Bergeron R, Shulman GI, Young LH . Translocation of myocardial GLUT4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 1999; 277: H643–H649.
Hopkins TA, Dyck JR, Lopaschuk GD . AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem Soc Trans 2003; 31: 207–212.
Saddik M, Gamble J, Witters LA, Lopaschuk GD . Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 1993; 268: 25836–25845.
Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR . Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation 2004; 109: 1550–1557.
Thampy KG . Formation of malonyl coenzyme A in rat heart. Identification and purification of an isozyme of A carboxylase from rat heart. J Biol Chem 1989; 264: 17631–17634.
Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD . Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol 1998; 275: H2122–H2129.
Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K et al. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J Biol Chem 2000; 275: 24279–24283.
Fischer Y, Thomas J, Sevilla L, Muñoz P, Becker C, Holman G et al. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem 1997; 272: 7085–7092.
Dennis SC, Gevers W, Opie LH . Protons in ischemia: where do they come from; where do they go to? J Mol Cell Cardiol 1991; 23: 1077–1086.
Liu B, Clanachan AS, Schulz R, Lopaschuk GD . Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res 1996; 79: 940–948.
Kantor PF, Dyck JR, Lopaschuk GD . Fatty acid oxidation in the reperfused ischemic heart. Am J Med Sci 1999; 318: 3–14.
Randle PJ, Garland PB, Newsholme EA, Hales CN . The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci 1965; 131: 324–333.
Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 upregulation in the late phase of cardioprotection. Cardiovasc Res 2004; 61: 610–619.
Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 2000; 10: 1247–1255.
Frederich M, Balschi JA . The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem 2002; 277: 1928–1932.
Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M et al. A pivotal role for endogenous TGF-beta-activated kinase-1 i the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 2006; 103: 17378–17383.
Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA et al. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem 1998; 273: 31880–31889.
Altarejos JY, Taniguchi M, Clanachan AS, Lopaschuk GD . Myocardial ischemia differentially regulates LKB1 and an alternate 5′-AMP-activated protein kinase kinase. J Biol Chem 2005; 280: 183–190.
Sakamoto K, Goransson O, Hardie DG, Alessi DR . Activity of LKB1 and AMPK-related kinases in skeletal muscle; effects of contraction, phenformin and AICAR. Am J Physiol Endocrinol Metab 2004; 287: E310–E317.
Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 2004; 10: 1384–1389.
Onay-Besikci A, Altarejos JY, Lopaschuk GD . gAd-globular head domain of adiponectin increases fatty acid oxidation in newborn rabbit hearts. J Biol Chem 2004; 279: 44320–44326.
Gamble J, Lopaschuk GD . Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism 1997; 46: 1270–1274.
Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR . Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem 2004; 278: 39422–39427.
Soltys CL, Kovacic S, Dyck JR . Activation of cardiac AMP-activated protein kinase (AMPK) by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am J Physiol Heart Circ Physiol 2006; 290: H2472–H2479.
Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of SER485/491. J Biol Chem 2006; 281: 5335–5340.
Longnus SL, Segalen C, Giudicelli J, Sajan MP, Farese RV, Van Obberghen E . Insulin signalling downstream of protein kinase B is potentiated by 5′AMP-activated protein kinase in rat hearts in vivo. Diabetologia 2005; 48: 2451–2453.
Young ME, Radda GK, Leighton B . Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR–an activator of AMP-activated protein kinase. FEBS Lett 1996; 382: 43–47.
Merrill GF, Kurth EJ, Hardie DG, Winder WW . AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 1997; 273: E1107–E1112.
Makinde AO, Gamble J, Lopaschuk GD . Upregulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res 1997; 80: 482–489.
Peralta C, Bartrons R, Serafin A, Blázquez C, Guzmán M, Prats N et al. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology 2001; 34: 1164–1173.
Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N et al. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res 2006; 98: 280–288.
Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem 2003; 278: 28372–28377.
Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC et al. Role of the alpha2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol 2006; 291: H2875–H2883.
Oliver MF, Kurien VA, Greenwood TW . Relation between serum-free-fatty acids and arrhythmias and death after acute myocardial infarction. Lancet 1968; 1: 710–714.
Mueller HS, Ayres SM . Metabolic response of the heart in acute myocardial infarction in man. Am J Cardiol 1978; 42: 363–371.
Lopaschuk GD, Collins-Nakai R, Olley PM, Montague TJ, McNeil G, Gayle M et al. Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J 1994; 128: 61–67.
Acknowledgements
GDL is an Alberta Heritage Foundation for Medical Research Medical Scientist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
Lopaschuk, G. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes 32 (Suppl 4), S29–S35 (2008). https://doi.org/10.1038/ijo.2008.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.120
Keywords
This article is cited by
-
Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction
Cardiovascular Diabetology (2019)
-
Differential effects of AMP-activated protein kinase in isolated rat atria subjected to simulated ischemia–reperfusion depending on the energetic substrates available
Pflügers Archiv - European Journal of Physiology (2018)
-
Coffee consumption, obesity and type 2 diabetes: a mini-review
European Journal of Nutrition (2016)
-
Pyrrolidinyl caffeamide against ischemia/reperfusion injury in cardiomyocytes through AMPK/AKT pathways
Journal of Biomedical Science (2015)
-
Direct effects of adipokines on the heart: focus on adiponectin
Heart Failure Reviews (2013)