Abstract
Context:
Hyperglycemia resolves quickly after bariatric surgery, but the underlying mechanism and the most effective type of surgery remains unclear.
Objective:
To examine glucose metabolism and β-cell function in patients with type 2 diabetes mellitus (T2DM) after two types of bariatric intervention; Roux-en-Y gastric bypass (RYGB) and gastric restrictive (GR) surgery.
Design:
Prospective, nonrandomized, repeated-measures, 4-week, longitudinal clinical trial.
Patients:
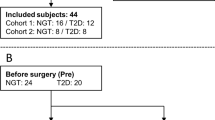
In all, 16 T2DM patients (9 males and 7 females, 52±14 years, 47±9 kg m−2, HbA1c 7.2±1.1%) undergoing either RYGB (N=9) or GR (N=7) surgery.
Outcome measures:
Glucose, insulin secretion, insulin sensitivity at baseline, and 1 and 4 weeks post-surgery, using hyperglycemic clamps and C-peptide modeling kinetics; glucose, insulin secretion and gut-peptide responses to mixed meal tolerance test (MMTT) at baseline and 4 weeks post-surgery.
Results:
At 1 week post-surgery, both groups experienced a similar weight loss and reduction in fasting glucose (P<0.01). However, insulin sensitivity increased only after RYGB, (P<0.05). At 4 weeks post-surgery, weight loss remained similar for both groups, but fasting glucose was normalized only after RYGB (95±3 mg 100 ml−1). Insulin sensitivity improved after RYGB (P<0.01) and did not change with GR, whereas the disposition index remained unchanged after RYGB and increased 30% after GR (P=0.10). The MMTT elicited a robust increase in insulin secretion, glucagon-like peptide-1 (GLP-1) levels and β-cell sensitivity to glucose only after RYGB (P<0.05).
Conclusion:
RYGB provides a more rapid improvement in glucose regulation compared with GR. This improvement is accompanied by enhanced insulin sensitivity and β-cell responsiveness to glucose, in part because of an incretin effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Defronzo RA . Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004; 88: 787–835.
Unger RH . Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 2003; 144: 5159–5165.
Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Defronzo RA . Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31–39.
Polonsky KS, Gumbiner B, Ostrega D, Griver K, Tager H, Henry RR . Alterations in immunoreactive proinsulin and insulin clearance induced by weight loss in NIDDM. Diabetes 1994; 43: 871–877.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724–1737.
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244: 741–749.
Cummings DE, Overduin J, Foster-Schubert KE . Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 2004; 89: 2608–2615.
Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299: 316–323.
Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006; 14: 1553–1561.
Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y bypass. Int J Obes 2009; 33: 786–795.
Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222: 339–350.
Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238: 467–484.
Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004; 240: 236–242.
Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 2007; 92: 4678–4685.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ . Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007; 3: 597–601.
Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687.
Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 2009; 94: 884–891.
Burguera B, Agusti A, Arner P, Baltasar A, Barbe F, Barcelo A et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J Endocrinol Invest 2007; 30: 844–852.
Defronzo RA, Tobin J, Andres R . Glucose clamp technique: a method for quantifying insulin secretion and insulin resistance. Am J Physiol 1979; 237: E214–E223.
O’Brien PE, Dixon JB, Laurie C, Anderson M . A prospective randomized trial of placement of the laparoscopic adjustable gastric band: comparison of the perigastric and pars flaccida pathways. Obes Surg 2005; 15: 820–826.
Roa PE, Kaidar-Person O, Pinto D, Cho M, Szomstein S, Rosenthal RJ . Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg 2006; 16: 1323–1326.
Van CE, Mestrez F, Sturis J, Polonsky KS . Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992; 41: 368–377.
Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006; 55: 2025–2031.
Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008; 8: 201–211.
Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002; 51: 2796–2803.
Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G . First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009; 32: 375–380.
Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO . Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol 1993; 48: M84–M90.
Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H . Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008; 135: 122–130.
Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK . Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 2003; 52: 1098–1103.
Guijarro A, Suzuki S, Chen C, Kirchner H, Middleton FA, Nadtochiv S et al. Characterization of weight loss and weight regain mechanisms following Roux-en-Y gastric bypass in rats. Am J Physiol Regul Intreg Comp Physiol 2007; 293: 1474–1489.
Johansson L, Roos M, Kullberg J, Weis J, Ahlstrom H, Sundbom M et al. Lipid mobilization following Roux-en-Y gastric bypass examined by magnetic resonance imaging and spectroscopy. Obes Surg 2008; 18: 1297–1304.
Bikman BT, Zheng D, Pories WJ, Chapman W, Pender JR, Bowden RC et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab 2008; 93: 4656–4663.
Mingrone G, DeGaetano A, Greco AV, Capristo E, Benedetti G, Castagneto M et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia 1997; 40: 599–605.
Phillips ML, Lewis MC, Chew V, Kow L, Slavotinek JP, Daniels L et al. The early effects of weight loss surgery on regional adiposity. Obes Surg 2005; 15: 1449–1455.
Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005; 11: 90–94.
Acknowledgements
We are grateful to the nurses and technicians in the Cleveland Clinic, Clinical Research Unit for providing the skilled assistance that enabled the successful implementation of this study. This work was supported in part by National Institutes of Health, National Center for Research Resources (NCRR), Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264 (SRK), National Institutes of Aging Award RO1 AG12834 (JPK), National Center for Research Resources, CTSA 1UL1RR024989 and by Ethicon Endo-Surgery (PRS, SRK). Grant support: NIH, NCRR, Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264, National Center for Research Resources, CTSA 1UL1RR024989 and Ethicon Endo-Surgery. Disclosure statement: Dr Kashyap discloses relationships with Ethicon endo-surgery (research grants); Dr Schauer discloses relationships with Ethicon endo-surgery (research grants).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary information
Rights and permissions
About this article
Cite this article
Kashyap, S., Daud, S., Kelly, K. et al. Acute effects of gastric bypass versus gastric restrictive surgery on β-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes 34, 462–471 (2010). https://doi.org/10.1038/ijo.2009.254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.254
Keywords
This article is cited by
-
Comparative impact of Roux-en-Y gastric bypass, sleeve gastrectomy or diet alone on beta-cell function in insulin-treated type 2 diabetes patients
Scientific Reports (2024)
-
Current status of metabolic surgery in patients with type I diabetes mellitus and obesity: a nationwide multicenter study
Langenbeck's Archives of Surgery (2023)
-
The Effects of Bariatric Surgery on Pharmacokinetics of Drugs: a Review of Current Evidence
Current Nutrition Reports (2023)
-
GLP-1: 10-year follow-up after Roux-en-Y gastric bypass
Langenbeck's Archives of Surgery (2022)
-
Änderungen der Betazellfunktion nach einer Magenbypassoperation
Der Diabetologe (2022)