Abstract
Objective:
To evaluate whether quantitative measures from magnetic resonance imaging (MRI) performed in hypothermia-treated encephalopathic newborns can differentiate patients with unfavorable neurological outcome.
Study Design:
Retrospective analysis of clinical data and MRI studies was performed in 47 full-term infants treated with whole-body hypothermia for neonatal encephalopathy. Apparent diffusion coefficients (ADCs) and T1 and T2 intensity ratios were measured in the basal ganglia and thalamus on axial MRI images. Unfavorable outcome was defined as (1) death or severe neurological deficits at discharge and (2) death or cerebral palsy/severe motor deficit at follow-up through age 9 months. Differences between groups with favorable versus unfavorable neurological outcome at each time point were compared. Optimal cutoff values for significant MR variables were determined with receiver operating curve analyses. Sensitivity and specificity of these cutoff values for predicting unfavorable outcome were calculated and results were compared with qualitative MRI interpretation.
Result:
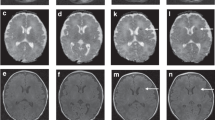
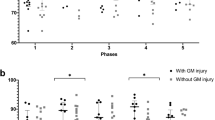
Infants presented with a median pH of 6.86, base deficit of 20 and Apgar scores of 1, 3 and 4 at 1, 5 and 10 min, respectively. Severe encephalopathy was present in 38%. Unfavorable outcome was present in 9 patients at discharge and in 13 of 26 patients with available follow-up data through 9 months. ADC values and T1 ratios were not significantly different between groups at either time point. T2 ratios in both the basal ganglia and thalamus were significantly higher in patients with unfavorable outcome, both at discharge and in follow-up. T2 intensity ratio in the basal ganglia and thalamus remained significantly associated with death or severe neurological deficit at discharge, after controlling for covariates in logistic regression analysis. Sensitivity and specificity of T2 intensity ratio for predicting unfavorable outcome at discharge were comparable to qualitative grading of injury in the basal ganglia and thalamus by a neuroradiologist.
Conclusion:
Increased T2 signal intensity in the basal ganglia or thalamus in patients with hypothermia-treated neonatal encephalopathy is associated with unfavorable neurological outcome at discharge and later with motor deficit/cerebral palsy. Quantitative methods to assess MRI evidence of brain injury are important for providing objective measures to predict outcome in this high-risk population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Robertson CMT, Finer NN, Grace MGA . School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr 1989; 114: 753–760.
Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R . Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev 1991; 25: 131–148.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF et al. Whole body hypothermia for neonates with hypoxic ischemic encephalopathy. N Engl J Med 2005; 353: 1574–1584.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomized trial. Lancet 2005; 365: 663–670.
Rutherford MA, Pennock JM, Schwieso JE, Cowan F, Dubowitz L . Hypoxic ischemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child 1996; 75: F145–F151.
Rutherford MA, Pennock JM, Counsell SJ, Mercuri E, Cowan FM, Dubowitz LM et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998; 102: 323–328.
Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. Am J Neuroradiol 1998; 19: 143–149.
Coskun A, Lequin M, Segal MM, Vigneron DB, Ferriero DM, Barkovich AJ . Quantitative analysis of MR images in asphyxiated neonates: correlation with neurodevelopmental outcome. Am J Neuroradiol 2001; 22: 400–405.
Miller SP, Newton N, Ferriero DM, Partridge JC, Glidden DV, Barnwell A et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatr Res 2002; 52: 71–77.
Rutherford MA, Pennock JM, Dubowitz LM . Cranial ultrasound and magnetic resonance imaging in hypoxic ischemic encephalopathy: a comparison with outcome. Develop Med Child Neurol 1994; 36: 813–825.
Blankenberg FG, Norbash AM, Lane B, Stevenson DK, Bracci PM, Enzmann DR . Neonatal intracranial ischemic and hemorrhage: diagnosis with US, CT and MR imaging. Radiology 1996; 199: 253–259.
Barkovich AJ . The encephalopathic neonate: choosing the proper imaging technique. Am J Neuroradiol 1997; 18: 1816–1820.
Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, Gouyon JB et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology 2006; 239: 839–848.
Wolf RL, Zimmerman RA, Clancy R, Haselgrove JH . Quantitative apparent diffusion coefficient measurements in term neonates for early detection of hypoxic-ischemic brain injury: initial experience. Radiology 2001; 218: 825–833.
Hunt RW, Neil JJ, Coleman LT, Kean MJ, Inder TE . Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics 2004; 114: 999–1003.
Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr 2004; 145: 835–837.
Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005; 116: 1001–1006.
Parikh NA, Lasky RE, Garza CN, Shankaran S, Tyson JE . Volumetric and anatomical MRI for hypoxic-ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments. J Perinatol 2009; 29: 143–149.
Volpe JJ . Hypoxic-ischemic encephalopathy: neuropathology and pathogenesis. In: Volpe JJ (ed). Neurology of the Newborn, 3rd edn. Saunders: Philadelphia, 1995; 279–313.
Barkovich AJ . MR and CT evaluation of profound neonatal and infantile asphyxia. Am J Neuroradiol 1992; 13: 959–972.
Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008; 121: 906–914.
Mercuri E, Rutherford M, Barnett A, Foglia C, Haataja L, Counsell S et al. MRI lesions and infants with neonatal encephalopathy. Is the Apgar score predictive? Neuropediatrics 2002; 33: 150–156.
Mehta CR, Patel NR . Exact logistic regression: theory and examples. Stat Med 1995; 14: 2143–2160.
Nelson KB, Ellemberg JH . Obstetrical complications as a risk factor for cerebral palsy or seizure disorders. JAMA 1994; 251: 1843.
Nelson KB . Relationship of intrapartum and delivery room events to long term neurologic outcome. Clin Perinatol 1989; 16: 995–1005.
Ruth VJ, Raivio KO . Perinatal brain damage: predictive value of metabolic acidosis and the Apgar score. Br Med J 1988; 297: 24–27.
Sarnat HB, Sarnat MS . Neonatal encephalopathy following fetal distress. Arch Neurol 1976; 33: 696–704.
Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007; 119: 912–921.
Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD et al. Predicting outcomes of neonates diagnosed with hypoxic ischemic encephalopathy. Pediatrics 2006; 118: 2084–2093.
Barkovich AJ, Westmark KD, Partridge C, Sola A, Ferriero DM . Perinatal asphyxia: MR findings in the first 10 days. Am J Neuroradiol 1995; 16: 427–438.
Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol 2006; 36: 582–592.
McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 2002; 59: 824–833.
Khong PL, Tse C, Wong IY, Lam BC, Cheung PT, Goh WH et al. Diffusion-weighted imaging and proton magnetic resonance spectroscopy in perinatal hypoxic-ischemic encephalopathy: association with neuromotor outcome at 18 months of age. J Child Neurol 2004; 19: 872–881.
Zarifi MK, Astrakas LG, Poussaint TY, duPlessis A, Zurakowski D, Tzika AA . Prediction of adverse outcome with cerebral lactate level and apparent diffusion coefficient in infants with perinatal asphyxia. Radiology 2002; 225: 859–870.
Acknowledgements
We acknowledge Cara H Olsen for her statistical consultation and analysis. We also thank William Gaillard for his insightful review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Massaro, A., Kadom, N., Chang, T. et al. Quantitative analysis of magnetic resonance images and neurological outcome in encephalopathic neonates treated with whole-body hypothermia. J Perinatol 30, 596–603 (2010). https://doi.org/10.1038/jp.2010.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2010.7
Keywords
This article is cited by
-
A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy
Pediatric Radiology (2017)
-
Prédiction du devenir neurologique à moyen terme par IRM cérébrale précoce, après encéphalopathie hypoxique-ischémique néonatale traitée par hypothermie contrôlée : apports de la séquence de diffusion et du calcul du coefficient apparent de diffusion (ADC), comparativement aux séquences morphologiques en pondération T1 et T2
Réanimation (2015)
-
The effect of whole-body cooling on brain metabolism following perinatal hypoxic–ischemic injury
Pediatric Research (2012)