Abstract
Objective:
To determine whether current retinopathy of prematurity (ROP) screening guidelines adequately identify treatable ROP in a contemporary cohort of extremely low gestation infants.
Study Design:
Data from the Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial were used. Inborn infants of 24 0/7 to 27 6/7 weeks gestational age (GA) with consent before delivery were enrolled in 2005 to 2009. Severe ROP (type 1 ROP or treatment with laser, cryotherapy or bevacizumab) or death was the primary outcome for the randomized trial. Examinations followed the then current AAP (American Academy of Pediatrics) screening recommendations, beginning by 31 to 33 weeks postmenstrual age (PMA).
Result:
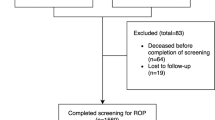
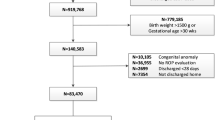
One thousand three hundred and sixteen infants were enrolled in the trial. Nine hundred and ninety-seven of the 1121 who survived to first eye exam had final ROP outcome determined. One hundred and thirty-seven (14% of 997) met criteria for severe ROP and 128 (93%) of those had sufficient data (without missing or delayed exams) to determine age of onset of severe ROP. PMA at onset was 32.1 to 53.1 weeks. In this referral center cohort, 1.4% (14/997) developed severe ROP after discharge.
Conclusion:
Our contemporary data support the 2013 AAP screening guidelines for ROP for infants of 24 0/7 to 27 6/7 weeks GA. Some infants do not meet treatment criteria until after discharge home. Post-discharge follow-up of infants who are still at risk for severe ROP is crucial for timely detection and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013; 131: 189–195.
American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2001; 108: 809–811.
American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006; 117: 572–576.
Jefferies A . Retinopathy of prematurity: recommendations for screening. Paediatr Child Health 2010; 15: 667–674.
Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB et al. Incidence and early course of retinopathy of prematurity. Ophthalmology 1991; 98: 1628–1640.
Reynolds JD, Dobson V, Quinn GE, Fielder AR, Palmer EA, Saunders RA et alfor the CRYO-ROP and LIGHT-ROP Cooperative Study Groups. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol 2002; 120: 1470–1476.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity; preliminary results. Pediatrics 1988; 81: 697–706.
Reynolds JD, Hardy RJ, Kennedy KA, Spencer R, van Heuven WA, Fielder AR . Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light Reduction in Retinopathy of Prematurity (LIGHT-ROP) Cooperative Group. N Engl J Med 1998; 338: 1572–1576.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR et alfor the NICHD Neonatal Research Network. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Ob Gynecol 2007; 196 (147): e1–e8.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126: 443–456.
The Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003; 121: 1684–1696.
Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M et alfor the Early Treatment for Retinopathy of Prematurity Cooperative Group. The incidence and course of retinopathy of prematurity: findings from the Early Treatment for Retinopathy of Prematurity Study. Pediatrics 2005; 116: 15–23.
Austeng D, Kallen KB, Hellstrom A, Tornqvist K, Holmstrom GE . Natural history of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch Ophthalmol 2010; 128: 1289–1294.
Isaza G, Arora S . Incidence and severity of retinopathy of prematurity in extremely premature infants. Can J Ophthalmol 2012; 47: 296–300.
Muether PS, Kribs A, Hahn M, Schumacher J, Eifinger F, Kirchhof B et al. No advanced retinopathy of prematurity stages 4 or 5 in a large high-risk German cohort. Br J Ophthalmol 2012; 96: 400–404.
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. New Engl J Med 2010; 362: 1959–1969.
American Academy of Pediatrics. An international classification of retinopathy of prematurity. Pediatrics 1984; 74: 127–133.
Hahn GJ, Meeker WQ . Statistical Intervals: A Guide for Practitioners. John Wiley & Sons: New York, NY, 1991.
Hussain N, Clive J, Bhandari V . Current incidence of retinopathy of prematurity, 1989-1997. Pediatrics 1999; 104: e26–e33.
Liu PM, Fang PC, Huang CB, Kou HK, Chung MY, Yang YH et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol 2005; 22: 115–120.
Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM . Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol 2009; 148: 451–458.
Phelps DL, Brown DR, Tung B, Cassady G, McClead RE, Purohit DM et al. 28-day survival rates of 6676 neonates with birth weights of 1250 grams or less. Pediatrics 1991; 87: 7–17.
Donovan EF, Tyson JE, Ehrenkranz RA, Verter J, Wright LL, Korones SB et al. Inaccuracy of Ballard scores before 28 weeks' gestation. J Pediatr 1999; 135: 147–152.
Rich W, Finer NN, Gantz MG, Newman NS, Hensman AM, Hale EC et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics 2012; 129: 480–484.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS . New intrauterine growth curves based on United States data. Pediatrics 2010; 125: e214–e224.
Walsh MC, Kliegman RM . Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986; 33: 179–201.
Acknowledgements
Data collected at participating sites of the National Institute of Child Health and Development Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC Principal Investigator), Dr Marie Gantz and Ms Wrage (DCC Statisticians) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904)—William Oh, MD; Betty R Vohr, MD; Angelita M Hensman, RN BSN; Bonnie E Stephens, MD; Barbara Alksninis, PNP; Dawn Andrews, RN; Kristen Angela, RN; Susan Barnett, RRT; Bill Cashore, MD; Melinda Caskey, MD; Kim Francis, RN; Dan Gingras, RRT; Regina A Gargus, MD FAAP; Katharine Johnson, MD; Shabnam Lainwala, MD; Theresa M Leach, MEd CAES; Martha R Leonard, BA BS; Sarah Lillie, RRT; Kalida Mehta; James R Moore, MD; Lucy Noel; Suzy Ventura; Rachel V Walden; Victoria E Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80)—Avroy A Fanaroff, MD; Deanne E Wilson-Costello, MD; Bonnie S Siner, RN; Arlene Zadell RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Hospital and Good Samaritan Hospital (U10 HD27853, M01 RR8084)—Edward F Donovan, MD; Vivek Narendran, MD MRCP; Kimberly Yolton, PhD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L Mincey, RN BSN; Jody Hessling, RN; Teresa L Gratton, PA.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center and Durham Regional Hospital (U10 HD40492, M01 RR30)—Ronald N Goldberg, MD; C Michael Cotten, MD MHS; Ricki F Goldstein, MD; Patricia Ashley, MD; Kathy J Auten, MSHS; Kimberley A Fisher, PhD FNP-BC IBCLC; Katherine A Foy, RN; Sharon F Freedman, MD; Kathryn E Gustafson, PhD; Melody B Lohmeyer, RN MSN; William F Malcolm, MD; David K Wallace, MD MPH.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital and Emory Crawford Long Hospital (U10 HD27851, RR25008, M01 RR39)—Barbara J Stoll, MD; Susie Buchter, MD; Anthony J Piazza, MD; David P Carlton, MD; Ira Adams-Chapman, MD; Linda Black, MD; Ann M Blackwelder, RNC BS MS; Sheena Carter, PhD; Elisabeth Dinkins, PNP; Sobha Fritz, PhD; Ellen C Hale, RN BS CCRC; Amy K Hutchinson, MD; Maureen Mulligan LaRossa, RN; Gloria V Smikle, PNP MSN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development—Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750)—Brenda B Poindexter, MD MS; Anna M Dusick, MD FAAP; James A Lemons, MD; Leslie D Wilson, BSN CCRC; Faithe Hamer, BS; Ann B Cook, MS; Dianne E Herron, RN; Carolyn Lytle, MD MPH; Heike M Minnich, PsyD HSPP.
National Heart, Lung, and Blood Institute—Mary Anne Berberich, PhD; Carol J Blaisdell, MD; Dorothy B Gail, PhD; James P Kiley, PhD.
RTI International (U10 HD36790)—W Kenneth Poole, PhD; Jamie E Newman, PhD MPH; Betty K Hastings; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; James W Pickett II, BS; Dennis Wallace, PhD; Kristin M Zaterka-Baxter, RN BSN.
Stanford University and Lucile Packard Children’s Hospital (U10 HD27880, UL1 RR25744, M01 RR70)—Krisa P Van Meurs, MD; David K Stevenson, MD; Susan R Hintz, MD MS Epi; M Bethany Ball, BS CCRC; Barbara Bentley, PsychD MSEd; Elizabeth F Bruno, PhD; Alexis S Davis, MD MS; Maria Elena DeAnda, PhD; Anne M DeBattista, RN, PNP; Jean G Kohn, MD MPH; Melinda S Proud, RCP; Renee P Pyle, PhD; Nicholas H St. John, PhD; Hali E Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54)—Ivan D Frantz III, MD; Elisabeth C McGowan, MD; John M Fiascone, MD; Anne Furey, MPH; Brenda L MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32)—Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V Collins, RN BSN MaEd; Shirley S Cosby, RN BSN; Vivien A Phillips, RN BSN; Kirstin J Bailey, PhD; Fred J Biasini, PhD; Maria Hopkins, PhD; Kristen C Johnston, MSN CRNP; Sara Krzywanski, MS; Kathleen G Nelson, MD; Cryshelle S Patterson, PhD; Richard V Rector, PhD; Leslie Rodriguez, PhD; Amanda Soong, MD; Sally Whitley, MA OTR-L FAOTA; Sheree York, PT DPT MS PCS.
University of California—San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461)—Maynard R Rasmussen, MD; Paul R Wozniak, MD; Yvonne E Vaucher, MD MPH; Wade Rich, RRT; Kathy Arnell, RNC; Rene Barbieri-Welge; Ayala Ben-Tall; Renee Bridge, RN; Clarence Demetrio, RN; Martha G Fuller, RN MSN; Elaine Ito; Meghan Lukasik; Deborah Pontillo; Donna Posin, OTR/L MPA; Cheryl Runyan; James Wilkes; Paul Zlotnik.
University of Iowa Children’s Hospital (U10 HD53109, UL1 RR24979, M01 RR59)—Edward F Bell, MD; John A Widness, MD; Michael J Acarregui, MD; Jonathan M Klein, MD; Tarah T Colaizy, MD MPH; Karen J Johnson, RN BSN; Diane L Eastman, RN CPNP MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587)—Shahnaz Duara, MD; Charles R Bauer, MD; Ruth Everett-Thomas, RN MSN; Maria Calejo, MEd; Alexis N Diaz, BA; Silvia M Frade Eguaras, BA; Andrea Garcia, MA; Kasey Hamlin-Smith, PhD; Michelle Harwood Berkowits, PhD; Sylvia Hiriart-Fajardo, MD; Elaine O Mathews, RN; Helina Pierre, BA; Arielle Riguard, MD; Alexandra Stroerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997)—Kristi L Watterberg, MD; Robin K Ohls, MD; Janell Fuller, MD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN; Jean Lowe, PhD; Rebecca Montman, BSN.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44)—Nirupama Laroia, MD; Gary David Markowitz, MD; Gary J Myers, MD; Linda J Reubens, RN CCRC; Diane Hust, MS RN CS; Lisa Augostino; Julie Babish Johnson, MSW; Erica Burnell, RN; Rosemary L Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Kelley Yost, PhD.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633)—Pablo J Sánchez, MD; Charles R Rosenfeld, MD; Walid A Salhab, MD; Roy J Heyne, MD; Sally S Adams, MS RN CPNP; James Allen, RRT; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T Heyne, PsyD PA-C; Melissa H Lepps, RN; Linda A Madden, RN CPNP; Melissa Martin, RN; Nancy A Miller, RN; Janet S Morgan, RN; Araceli Solis, RRT; Lizette E Torres, RN; Catherine Twell Boatman, MS CIMI; Diana M Vasil, RNC-NIC; Kerry Wilder, RN.
University of Texas Health Science Center at Houston Medical School and Children’s Memorial Hermann Hospital (U10 HD21373)—Jon E Tyson, MD MPH; Patricia W Evans, MD; Nora I Alaniz, BS; Patricia Evans, MD; Beverly Foley Harris, RN BSN; Charles Green, PhD; Margarita Jiminez, MD MPH; Anna E Lis, RN BSN; Sarah Martin, RN BSN; Georgia E McDavid, RN; Brenda H Morris, MD; Margaret L Poundstone, RN BSN; Stacy Reddoch, BA; Saba Siddiki, MD; Patti L Pierce Tate, RCP; Laura L Whitely, MD; Sharon L Wright, MT (ASCP).
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64)—Anna Bodnar, MD; Shawna Baker, RN; Karie Bird, RN; Jill Burnett, RN; Laura Cole, RN; Karen A Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Mike Steffens, PhD; Kimberlee Weaver-Lewis, RN BSN; Karen Zanetti, RN.
Wake Forest University, Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122)—T Michael O’Shea, MD MPH; Robert G Dillard, MD; Lisa K Washburn, MD; Nancy J Peters, RN CCRP; Barbara G Jackson, RN BSN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L Waldrep, MS; Cherrie D Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385)—Seetha Shankaran, MD; Beena G Sood, MD MS; Athina Pappas, MD; Rebecca Bara, RN BSN; Elizabeth Billian, RN MBA; Laura A Goldston, MA; Mary Johnson, RN BSN.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, MO1 RR125)—Richard A Ehrenkranz, MD; Vineet Bhandari, MD DM; Harris C Jacobs, MD; Pat Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Christine G Butler, MD; Nancy Close, PhD; Walter Gilliam, PhD; Sheila Greisman, RN; Elaine Romano, MSN; Joanne Williams, RN BSN.
Data and Safety Monitoring Committee—Gordon Avery, MD, chair, Children’s National Medical Center; Christine A Gleason, MD, chair, University of Washington; Marilee C Allen, MD, Johns Hopkins University School of Medicine; Shrikant I Bangdiwala, PhD, University of North Carolina; Carol J Blaisdell, MD, National Heart, Lung, and Blood Institute; Robert J Boyle, MD, University of Virginia Health System; Traci Clemons, PhD, The EMMES Corporation; Mary E D’Alton, MD, Columbia University; Abhik Das (ex officio), PhD, RTI International; Dorothy B Gail, PhD, National Heart, Lung, and Blood Institute; Carl Hunt, MD, National Heart, Lung, and Blood Institute; Martin Keszler, MD, Georgetown University Hospital; W Kenneth Poole (ex officio), PhD, RTI International; Carol K Redmond, ScD, University of Pittsburg; Michael G Ross, MD, MPH; UCLA School of Medicine and Public Health; Merran A Thomson, MD, Hammersmith Hospital, UK; Steven J Weiner, MS, The George Washington University; Marian Willinger (ex officio), PhD, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Retinopathy of Prematurity Adjudication Committee—Gary David Markowitz, MD, University of Rochester; Amy K Hutchinson, MD, Emory University; David K Wallace, MD, MPH, Duke University; Sharon F Freedman, MD, Duke University.
Funding source: The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network’s SUPPORT trial.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kennedy, K., Wrage, L., Higgins, R. et al. Evaluating retinopathy of prematurity screening guidelines for 24- to 27-week gestational age infants. J Perinatol 34, 311–318 (2014). https://doi.org/10.1038/jp.2014.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.12
Keywords
This article is cited by
-
National guideline for ophthalmological screening of premature infants in Germany (S2k level, AWMF guidelines register no. 024/010, March 2020)
Die Ophthalmologie (2022)
-
Augenärztliche Screening-Untersuchung bei Frühgeborenen (S2k-Level, AWMF-Leitlinien-Register-Nr. 024/010, März 2020)
Der Ophthalmologe (2021)
-
Prenatal and postnatal inflammation-related risk factors for retinopathy of prematurity
Journal of Perinatology (2019)