Abstract
The objective of this review is to assess the effect of home-based neonatal care provided by community health workers (CHWs) for preventing neonatal, infant and perinatal mortality in resource-limited settings with poor access to health facility-based care. The authors conducted a systematic review, including meta-analysis and meta-regression of controlled trials. The data sources included electronic databases, with a hand search of reviews, abstracts and proceedings of conferences to search for randomized, or cluster randomized, controlled trials evaluating the effect of home-based neonatal care provided by CHWs for preventing neonatal, infant and perinatal mortality. Among the included trials, all from South Asian countries, information on neonatal, infant and perinatal mortality was available in five, one and three trials, respectively. The intervention package comprised three components, namely, home visits during pregnancy (four trials), home-based preventive and/or curative neonatal care (all trials) and community mobilization efforts (four trials). Intervention was associated with a reduced risk of mortality during the neonatal (random effects model relative risk (RR) 0.75; 95% confidence intervals (CIs) 0.61 to 0.92, P=0.005; I2=82.2%, P<0.001 for heterogeneity; high-quality evidence) and perinatal periods (random effects model RR 0.78; 95% CI 0.64 to 0.94, P=0.009; I2=79.6%, P=0.007 for heterogeneity; high-quality evidence). In one trial, a significant decline in infant mortality (RR 0.85; 95% CI 0.77 to 0.94) was documented. Subgroup and meta-regression analyses suggested a greater effect with a higher baseline neonatal mortality rate. The authors concluded that home-based neonatal care is associated with a reduction in neonatal and perinatal mortality in South Asian settings with high neonatal-mortality rates and poor access to health facility-based care. Adoption of a policy of home-based neonatal care provided by CHWs is justified in such settings.
Similar content being viewed by others
Introduction
The last three decades have witnessed a significant fall in infant mortality rates in developing countries, whereas neonatal mortality rates have decreased at a slower pace.1, 2 Estimates published in 2008 suggest that about 41% of all under-five mortality occurs in the neonatal period,3 contributing four million deaths worldwide each year.4 Nearly all (99%) global neonatal mortality occurs in developing countries.3 Lowering this mortality is vital for achieving further reductions in infant and child mortality.1, 5, 6, 7, 8
Among neonatal deaths, three quarters occur during the first week of life whereas 25 to 45% occur within the first 24 h. The majority of neonatal deaths happen at home against a backdrop of rural poverty, unskilled neonatal care and a suboptimal or absent referral system; a strategy that promotes universal access to antenatal care, skilled birth attendance and early postnatal care has the potential to contribute to a sustained reduction in neonatal mortality.1, 5 A complementary approach is community-based delivery of key newborn health interventions. Two related modalities have been attempted in programs and research trials in the last decade. The first approach involves home visits and other community activities for the promotion of optimal newborn-care practices. The second approach, in addition to the promotion of preventive interventions, includes home-based management of perinatal and neonatal morbidities such as birth asphyxia and neonatal sepsis.
Since utilization of health facilities for neonatal health is low, the potential complementary role for home-based newborn care in accelerating the decline in neonatal deaths to achieve Millennium Development Goal 4 needs to be assessed. Recent reviews have evaluated the efficacy of community-based interventions, including home-based neonatal care, in reducing neonatal mortality.9, 10 In these reviews, the relative paucity of eligible trials necessitated the inclusion of non-randomized or quasi-randomized trials, which partially compromised the quality of synthesized evidence. Following the recent publication of randomized controlled trials, updating the available systematic reviews to guide relevant policy is necessary.
The objective of this review is to assess the effect of home-based neonatal care provided by community health workers (CHWs) for preventing neonatal mortality in resource-limited settings with poor access to health facility-based care.
Methods
Criteria for considering trials for this review included the following:
Types of trials
Trials evaluating home-based neonatal care provided by CHWs with a concurrent control group and a random design, with individual or cluster allocation, were eligible for inclusion. Trials primarily evaluating home-based neonatal care following birth in a facility or hospital were excluded.
Types of participants
The trial population comprised neonates (first 28 days of life, or the first month of life where not specified in days) born in resource-limited settings with poor access to health facility-based care.
Types of interventions
Experimental interventions comprised promotion of optimal neonatal care practices at home, with or without home-based treatment of neonatal morbidities, delivered by CHWs during the neonatal period, with or without additional interventions during pregnancy and/or childbirth. The experimental intervention was compared with controls who did not receive any home-based intervention by CHWs during the neonatal period.
Interventions during pregnancy included: (i) promotion of antenatal care; (ii) health education and/or counseling of the mother regarding desirable practices during pregnancy; or (iii) promotion of delivery in a hospital or at home by a skilled birth attendant.
Interventions during childbirth included: (i) education about safe and/or clean delivery practices; or (ii) implementation of safe delivery practices in case of domiciliary deliveries.
Interventions during the neonatal period consisted of: (i) care of the newborn immediately after birth, including keeping the baby warm, neonatal resuscitation (if required) and early initiation of breastfeeding; (ii) health education and/or counseling of families regarding neonatal care practices such as exclusive breastfeeding, keeping the baby warm and hygienic cord care; (iii) education to improve caregiver recognition of life-threatening neonatal problems; (iv) education to improve health care-seeking behaviors; (v) identification of signs of severe neonatal morbidities and referral to a health facility; or (vi) home-based management of neonatal morbidities.
The term ‘community health worker’ included any of the following personnel: village or CHWs or volunteers (paid/unpaid), public health nurse or auxiliary nurse.
Types of outcome measures
Primary outcomes
All-cause mortality included: (i) neonatal deaths due to any cause during the period between initiation of the intervention and the last follow-up within the first month of life; and (ii) infant deaths due to any cause during the period between initiation of the intervention and the last follow-up within the first year of life.
Secondary outcomes
These secondary outcomes included: (i) perinatal mortality rate; and (ii) cause-specific mortality including deaths due to neonatal sepsis, tetanus, asphyxia and prematurity (as defined by the authors, irrespective of single- or multiple-cause assignment).
Search methods for identification of trials
We searched computerized bibliographic medical databases, including Medline, Cochrane Controlled Trials Register in the Cochrane Library, EMBASE, Health Services Technology, Administration, and Research (HealthSTAR) and clinical trials websites through 5 May 2012. For PubMed the following search strategy was used:
(newborn or neonat* OR peri-natal) AND (‘community’ OR community-based OR home OR home-based OR domiciliary OR rural OR traditional OR village OR village-based) AND (mortality OR death OR survival OR outcome) AND (Clinical Trial[ptyp] OR Randomized Controlled Trial[ptyp] OR Controlled Clinical Trial[ptyp] OR Evaluation Studies[ptyp] OR Journal Article[ptyp]) AND (infant [MeSH]) AND (Humans[Mesh]).
A lateral search using the link of related articles in PubMed was done for articles initially selected from the search strategy. We also reviewed the reference lists of identified articles and hand-searched reviews, bibliographies of books and abstracts and proceedings of international conferences and meetings. Experts in the field were contacted to identify any additional or ongoing trials. The title and abstract of the trials identified in the computerized search were scanned to exclude trials that were obviously irrelevant. Full texts of the identified trials that fulfilled the inclusion criteria were reviewed. To avoid publication bias, we attempted to include both published and unpublished trials.
Quality assessment
In order to enhance the validity of the meta-analysis, the quality of the identified trials was assessed by Cochrane Collaboration’s tool for assessing the risk of bias.11 This tool assesses the degree to which: (i) the allocation sequence was adequately generated (sequence generation); (ii) the allocation was adequately concealed (allocation concealment); (iii) knowledge of the allocated interventions was adequately prevented during the study (blinding); (iv) incomplete outcome data were adequately addressed; (v) reports of the study were free of suggestion of selective outcome reporting; and (vi) the study was apparently free of other problems that could put it at high risk of bias (for example, conflict of interest, premature trial termination). Each domain was allocated one of the three possible categories for each of the included studies: ‘Yes’ for low risk of bias, ‘No’ for high risk of bias and ‘Unclear’ where the risk of bias was uncertain or unknown.
Data abstraction
Data abstraction was done in duplicate using a standard questionnaire. The data included in the review were derived from the published manuscript or as provided by the authors for unpublished trials. Requests to the original investigators for additional data and information were made if required. Data entry and initial analysis were performed on SPSS (IBM, Armonk, NY, USA) (Version 14.0) software.12
Analysis
Meta-analysis was performed with a user-written program on STATA (version 9.2) software (StataCorp, College Station, TX, USA).13 The presence of bias in the extracted data was evaluated quasi-statistically using a funnel plot.14 The effect measure was plotted against the standard error of the effect size on a log scale. In the absence of bias, because of the sampling variability, the graph takes the form of an inverted funnel. In the presence of bias, the corner of the funnel is distorted or missing. Formal statistical tests for funnel plot asymmetry, namely the Begg’s and Egger’s methods, were also conducted with the user-written ‘metabias’ command in STATA (version 9.2) software.15, 16 Pooled estimates (relative risk (RR) with 95% confidence intervals (CIs)) of the evaluated outcome measures were calculated by the generic inverse variance method by the user-written ‘metan’ command15, 17 in STATA (version 9.2) software. The natural logarithm converted values of the individual trial RRs, and their standard errors were used for computing the pooled estimates as recommended.17 These pooled estimates were expressed in an exponential form. This program also computes formal tests of heterogeneity, namely, the statistic Cochran Q and I2 (variation in pooled estimate attributable to heterogeneity).
One option for analyzing the data was to calculate the change in mortality rates (from baseline to the end of the intervention or observation period) in the intervention and control groups separately, and then construct RRs and 95% CIs for the difference in the change between the two groups. The other option was to calculate the RR and 95% CIs on the basis of a comparison of mortality rates at the end of the intervention or observation period in the intervention and control groups. We utilized the second option because baseline and/or change data were not available for all included trials. For computing the summary RR, we required individual trial RR and 95% CI or standard error. In the case of cluster-randomized trials citing cluster-adjusted values, we used the reported values. For trials reporting only cluster-specific data, we used a random-effects version of the STATA procedure XTLOGIT to derive an odds ratio, allowing for the cluster design. Random effects were fitted for each village, and the odds ratio was used to give an estimate of RR in the rare outcomes we were modeling.
The outcome variables were pooled using both fixed-effects and random-effects model assumptions. No comprehensive rules exist on when to use these models; debate continues in the statistical community. The underlying assumption for the fixed-effects model is that each trial estimates the same true population value for the effect of interest, and thus the differences between observed results of trials can be accounted for fully by sampling variation. Random-effects models assume that a distribution of population effects exists and is generated by a distribution of possible trial effect situations. Thus, outcomes of trials may differ both because of sampling variation and true differences in effects. Both random- and fixed-effects models can be appropriately applied to pooling of data and also for evaluating the sensitivity of results to differing model assumptions. The random-effects model is generally preferred in the presence of significant heterogeneity.
Sensitivity and subgroup analyses
Sensitivity and sub-group analyses were performed only for the primary outcome, all-cause neonatal mortality, to explore heterogeneity and also as a hypothesis-generating exercise. The following pre-specified sensitivity and subgroup analyses were performed: (i) preventive interventions versus preventive and curative interventions (antibiotics for neonatal sepsis) to examine the potential effect of adding curative treatment; (ii) high (>50 neonatal deaths per 1000 live births) versus low (⩽50 neonatal deaths per 1000 live births) baseline neonatal mortality (derived from the control group) to examine the possibility of a greater benefit in populations with higher baseline mortality; (iii) proportion of neonates receiving a postnatal visit (<50% versus ⩾50%) to examine the effect of the extent of coverage on mortality; and (iv) various elements of risk of bias assessment (low risk versus unclear and high risk). The contribution of these variables to heterogeneity was also explored by meta-regression using the ‘metareg’ command in STATA (version 9.2) software with the restricted maximum likelihood option.18
Results
Trial flow
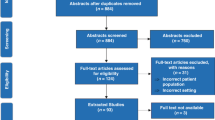
A total of 85 potentially eligible references were identified.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103 Among these, 77 references were excluded19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 95, 97 (Figure 1). The reasons for excluding these references are detailed in Table 1. The remaining eight references, which pertained to five trials, were included in the review.94, 96, 98, 99, 100, 101, 102, 103
Trial characteristics
Table 2 summarizes the characteristics of included trials. All five trials were conducted in South Asia, and all were cluster-randomized trials, which provided cluster-adjusted mortality data.
Intervention package
Table 3 summarizes the CHW characteristics and intervention package used in the included trials. Substantial heterogeneity was evident for these aspects.
Training and supervision of health workers
Table 4 summarizes the duration and content of training provided to the CHWs delivering the intervention in the respective trials. Substantial heterogeneity was evident for these aspects.
Risk of bias assessment
The risk of bias assessment for these trials is detailed in Table 5 and depicted graphically in Figure 2. Except for an unclear risk of selection bias in one trial, all studies were assessed to be at low risk of bias for all elements.
Quantitative data synthesis
All five trials provided neonatal mortality data,94, 96, 98, 99, 100, 101, 102, 103 and three trials provided perinatal mortality data.96, 100, 101 One trial provided infant mortality data,100 and one trial provided cause-specific mortality data.102
The Shivgarh (India) trial96 had two very similar intervention groups, with home-based essential newborn care as the core intervention. One intervention group additionally used a technology called ‘Thermospot’ to help caregivers decide if their newborn’s temperature was low. We therefore excluded the intervention arm with ‘Thermospot’ and used the data provided by the authors comparing only the home-based neonatal care group with the control group.
The Sylhet (Bangladesh) trial99 also had two intervention arms, one called ‘home care’ and the other ‘community care’. We excluded the ‘community care’ arm from the analysis because the interventions in this arm did not meet the inclusion criteria for this review.
Neonatal mortality
All five trials provided neonatal mortality data.94, 96, 98, 99, 100, 101, 102, 103 The funnel plot (Figure 3) appeared symmetrical, and there was no evidence of significant (P=0.204) bias with the Egger’s (weighted regression) method. The intervention was associated with a reduced risk of mortality during the neonatal period; the pooled relative risk was 0.75 (95% CI 0.61 to 0.92, P=0.003; I2=82.2%, P<0.001) by random effects model (Figure 4) and 0.82 (95% CI 0.76 to 0.89, P<0.001) by fixed-effects model.
On performing pre-specified sensitivity and subgroup analyses (Table 6) significant heterogeneity was suggested only with higher baseline neonatal mortality. Trials with a baseline rate of more than 50/1000 live births had a significantly greater reduction in neonatal mortality (RR 0.46; 95% CI 0.35 to 0.60; P<0.001), compared with trials with a baseline rate <50/1000 live births (RR 0.86; 95% CI 0.79 to 0.94, P<0.001; heterogeneity P<0.001) (Figure 5). There was no evidence of significant heterogeneity in the other two pre-specified subgroups, namely, the coverage of home visits (Figure 6) and the type of care (Figure 7). The details of program coverage and curative treatment offered in various trials are depicted in Tables 7 and 8, respectively. The pre-specified sensitivity analyses for bias could not be performed because, except for an unclear risk of selection bias in one trial, all studies were assessed to be at low risk of bias for all elements.
On performing univariate meta-regression (Table 9) analyses, none of the variables emerged as significant predictors of heterogeneity. However, baseline neonatal mortality approached conventional statistical significance (P=0.065).
We conducted a post hoc sensitivity analysis by combining evidence from three23, 24, 25, 95, 97 non-randomized or quasi-randomized trials with concurrent control groups (Figure 8). There was no evidence of significant heterogeneity (P=0.192) for the comparison between randomized and non-randomized trials. With the random effects model, the RR for randomized trials was 0.75 (95% CI 0.61 to 0.92; I2=82.2%) and for non-randomized trials was 0.67 (95% CI 0.40 to 1.13; I2=89.5%). The overall effect size with inclusion of all eight trials was 0.72 (95% CI 0.60 to 0.87; I2=83.8%).
Infant mortality
Data were available from one trial that showed a significant decline in infant mortality with RR of 0.85 (95% CI 0.77 to 0.94).100
Perinatal mortality rate
Data were pooled from three trials.96, 100, 101 There was evidence of a reduced risk of perinatal mortality; the pooled RR was 0.78 (95% CI 0.64 to 0.94, P=0.009; I2=79.6%, P=0.007) by random-effects model (Figure 9). A similar result was obtained with the fixed-effects model.
Cause-specific mortality
Only one trial provided cause-specific mortality data in neonates in the form of rates in each comparison group without cluster-adjustment RRs.102
Summary of findings
The GRADE summary of findings is shown in Table 10. The quality of evidence was graded as high for neonatal and perinatal mortality, and moderate for infant mortality.
Discussion
This systematic review of five cluster-randomized trials indicates that home-based neonatal care provided by CHWs is associated with significant neonatal mortality reduction in resource-limited settings with poor access to health facility-based care (high-quality evidence). Data from three trials indicated a reduction in the perinatal mortality rate (high-quality evidence). There was evidence of a reduction in infant mortality in the only trial providing this information. The baseline neonatal mortality rate emerged as a potential predictor of the neonatal mortality effect.
Strengths and limitations of analyses
This updated systematic review incorporated relevant subgroup and meta-regression analyses, and there was no evidence of publication bias. All cluster-randomized trials were appropriately combined by design effect correction for mortality outcomes. This review represents the synthesis of the most contemporary evidence for translating into public health policy as all the included trials were published within the past 5 years.
Some limitations merit consideration. First, data on perinatal mortality was limited to three trials, whereas only one trial reported infant mortality and cause-specific mortality. Second, all trials were conducted in South Asia, which limits generalizing to similar settings in other continents, particularly sub-Saharan Africa. Trials evaluating community-based interventions without a specific element of home-based neonatal care delivered by a CHW were excluded because such data had different programmatic implications from the policy under consideration.
The findings of this systematic review are in conformity with two earlier reviews on this subject, which were not restricted to randomized trials. We included only randomized controlled trials to aim for the highest quality of evidence. However, the main findings remained stable in a post hoc sensitivity analysis combining the evidence from three additional non-randomized or quasi-randomized trials with concurrent controls (Figure 8).
As noted earlier, no comprehensive rules exist for when to use random effects or fixed effects models for meta-analysis. Fixed-effects analysis is appropriate if there is a reasonable assumption that the trials are estimating the same underlying treatment effect (that is, they are similar enough in their populations, interventions and methods to make this plausible). Random-effects analysis assumes a distribution of effect sizes, and it estimates the center of that distribution and the uncertainty around it. It is more appropriate for situations where there are differences in design, population or intervention between included trials that may be sufficient to affect their treatment effects. We preferred the random-effects model because of significant contextual differences in included trials and documentation of statistical heterogeneity (I2>50%) for neonatal mortality. However, the estimates from the random- and fixed-effects models were in broad conformity (Table 6).
Subgroup analyses and meta-regression suggested a greater survival benefit in settings with higher baseline neonatal mortality rates. Home-based neonatal care interventions are primarily effective in reducing neonatal sepsis and mild asphyxia. As the neonatal mortality rate decreases in an area, the cause-specific mortality due to sepsis decreases and asphyxia probably remains unchanged, whereas the proportion of mortality due to preterm births (as well as the absolute number) increases. In the Mirzapur trial102 (baseline neonatal mortality rate 27.9/1000 live births), nearly 60% of deaths were due to birth asphyxia or prematurity; the program had limitations in reaching households at critical times (that is, during labor, childbirth and immediately after delivery) to address these conditions, whereas the CHWs lacked the necessary tools and skills to effectively attend to them. Unfortunately, the other trials did not provide cause-specific mortality to explore this possibility. In settings with lower baseline neonatal mortality rates, there may be a greater role of community mobilization and effective referral to facility-based care to address these causes of death.
Program coverage did not emerge as a significant predictor of the decrease in the neonatal mortality effect. However, program coverage was defined by the number of live births receiving a postnatal home visit in the first 48 to 72 h. Hence, it does not encapsulate the whole construct of the intervention that the trials had employed; many trials had excellent community mobilization programs in spite of low coverage of postnatal visits.101 Furthermore, with a sample size of five trials, the analysis had limited power to detect a positive predictor.
The addition of a curative component (antibiotics for neonatal sepsis) to the intervention did not emerge as a significant predictor of neonatal mortality. No included trial provided for treatment of birth asphyxia by CHWs as part of the home-based package of neonatal care, and it is unclear whether providing training and equipment to CHWs reduces mortality due to asphyxia.68, 104 As CHWs are likely to encounter asphyxia only sporadically, continued training for maintenance of skills to manage it may be challenging.
In all the trials under review, the intervention was delivered as a package comprising three components, namely, home visits during pregnancy (four trials), home-based neonatal care (all trials) and community mobilization efforts (four trials). The reduction in neonatal and perinatal mortality cannot therefore be solely ascribed to the home-based neonatal care component. However, from a programmatic perspective this is not crucial; in practice antenatal visits would be required to establish contact with pregnant women for postnatal visits, and health workers can also perform some community mobilization services.
Implications for policy
Home-based neonatal care is associated with reductions in neonatal and perinatal mortality in settings with high neonatal mortality rates and poor access to health facility-based care. The high-quality evidence in this review thus provides support for adopting a policy of home-based neonatal care provided by CHWs in such settings. Concrete recommendations cannot be made regarding the optimal timing of home visits and specific responsibilities of CHWs. However, data suggest that antenatal visits and home-based neonatal care within the first week of life should be an integral part of this intervention. Incorporating a component of community mobilization in addition to home-based neonatal care would be desirable. All the evidence pertains to South Asia; however, there are no obvious reasons to suspect different results in other regions with similar neonatal mortality rates and access to health care.
Implications for future research
The following gaps in evidence should be addressed as a priority to provide further directions for policy: (i) efficacy of the intervention package in similar settings in other regions, particularly sub-Saharan Africa; (ii) evaluating the benefit of adding treatment of sepsis and birth asphyxia; (iii) the effect of the intervention package on infant and cause-specific mortality; and (vi) operational research in pilot programs to evaluate coverage levels and quality, reasons for poor performance and possible interventions for improvement.
Concluding comments
Home-based neonatal care is associated with reductions in neonatal and perinatal mortality (high-quality evidence) in South Asian settings with high neonatal-mortality rates and poor access to health facility-based care. Adopting a policy of home-based neonatal care provided by CHWs is justified in such settings.
References
Darmstadt G, Lawn J, Costello A . Advancing the state of the world's newborns. Bull World Health Organ 2003; 81: 224–225.
Hyder A, Morrow R, Wali S, McGuckin J . Burden of Disease for Neonatal Mortality in South Asia and Sub-Saharan Africa. Save the Children Federation–USA: Washington, DC, USA, 2001.
Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375 (9730): 1969–1987.
Lawn J, Cousens S, Zupan J . 4 million neonatal deaths: When? Where? Why? Lancet 2005; 365: 891–900.
Darmstadt G, Black R, Santosham M . Research priorities and postpartum-care strategies for the prevention and treatment of neonatal infections in less developed countries. Pediatr Infect Dis J 2000; 19: 739–750.
Child Health Research Project Reducing Perinatal and Neonatal Mortality. Johns Hopkins School of Public Health: Baltimore. MD, USA, 1999.
Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L . Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 2005; 365: 977–988.
Moss W, Darmstadt G, Marsh D, Black R, Santosham M . Research priorities for the reduction of perinatal and neonatal morbidity and mortality in developing country communities. J Perinatol 2002; 22: 484–495.
Gogia S, Sachdev HS . Home visits by community health workers to prevent neonatal deaths in developing countries: a systematic review. Bull World Health Organ 2010; 88: 658–666.
Gogia S, Ramji S, Gupta P, Gera T, Shah D, Mathew JL et al. Community based newborn care: a systematic review and meta-analysis of evidence: UNICEF-PHFI series on newborn and child health, India. Indian Pediatr 2011; 48: 537–546.
Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available at www.cochrane-handbook.org.
SPSS SPSS for Windows, Release 14.0.0. SPSS Inc.: Chicago, IL, USA, 2005.
StataCorp Stata Statistical Software, Release 9. StataCorp LP: College Station, TX, USA, 2005.
Sterne JAC, Egger M, Smith GD . Investigating and dealing with publication and other biases. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books: London, UK, 2001, pp 189–208.
Sterne JAC, Bradburn MJ, Egger M Meta-analysis in STATA TM. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care: Meta-analysis in Context. BMJ Books: London, UK, 2001, pp 347–369.
Steichen TJ, Egger M, Sterne JAC . sbe19.1: tests for publication bias in meta-analysis. Stata J 1998; 44: 3–4.
Harris R, Bradburn MJ, Deeks J, Harbord R, Altman D, Steichen T et al Stata Version 9 Update (Distribution Date 19 February 2007) for the Stata User Written Programme sbe24. http://fmwww.bc.edu/RePEc/bocode/m, accessed on 24 March 2007.
Harbord R, Steichen T, Sharp SJ . ‘metareg: Update to Stata Module to Perform Meta-Analysis Regression’. Statistical Software Components S4446201, Boston College Department of Economics, revised 02 February 2005, http://ideas.repec.org/c/boc/bocode/s446201.html accessed on 15 March 2007.
Osrin D, Mesko N, Shrestha B, Shrestha D, Tamang S, Thapa S et al. Implementing a community-based participatory intervention to improve essential newborn care in rural Nepal. Trans R Soc Trop Med Hyg 2003; 97: 18–21.
Pratinidhi A, Shah U, Shrotri A, Bodhani N . Risk approach strategy in neonatal care. Bull World Health Organ 1986; 64: 291–297.
Greenwood A, Bradley A, Byass P, Greenwood BM, Snow RW, Bennett S et al. Evaluation of a primary health care programme in the Gambia. I. The impact of trained traditional birth attendants on the outcome of pregnancy. J Trop Med Hyg 1990; 93: 58–66.
Alisjahbana A, Williams C, Dharmayanti R, Hermawan D, Kwast BE, Koblinsky M . An integrated village maternity service to improve referral patterns in a rural area in West-Java. Int J Gynaecol Obstet 1995; 48 (Suppl): S83–S94.
Bang AT, Bang RA, Tale O, Sontakke P, Solanki J, Wargantiwar R et al. Reduction in pneumonia mortality and total childhood mortality by means of community-based intervention trial in Gadchiroli, India. Lancet 1990; 336: 201–206.
Bang AT, Bang RA, Morankar VP, Sontakke PG, Solanki JM . Pneumonia in neonates: can it be managed in the community? Arch Dis Child 1993; 68: 550–556.
Bang AT, Bang RA, Sontakke PG . Management of childhood pneumonia by traditional birth attendants. The SEARCH Team. Bull World Health Organ 1994; 72: 897–905.
Bilenko N, Hammel R, Belmaker I . Utilization of antenatal care services by a semi-nomadic Bedouin Arab population: evaluation of the impact of a local maternal and child health clinic. Matern Child Health J 2007; 11: 425–430.
Bolam A, Manandhar DS, Shrestha P, Ellis M, Costello AM . The effects of postnatal health education for mothers on infant care and family planning practices in Nepal: a randomised controlled trial. BMJ 1998; 316: 805–811.
Daga SR, Daga AS, Dighole RV, Patil RP, Dhinde HL . Rural neonatal care: Dahanu experience. Indian Pediatr 1992; 29: 189–193.
Daga SR, Daga AS, Dighole RV, Patil RP . Anganwadi worker's participation in rural newborn care. Indian J Pediatr 1993; 60: 627–630.
de Francisco A, Schellenberg JA, Hall AJ, Greenwood AM, Cham K, Greenwood BM . Comparison of mortality between villages with and without primary health care workers in Upper River Division, The Gambia. J Trop Med Hyg 1994; 97: 69–74.
Edgerley LP, el-Sayed YY, Druzin ML, Kiernan M, Daniels KI . Use of a community mobile health van to increase early access to prenatal care. Matern Child Health J 2007; 11: 235–239.
Fauveau V, Stewart K, Khan SA, Chakraborty J . Effect on mortality of community-based maternity-care programme in rural Bangladesh. Lancet 1991; 338: 1183–1186.
Foord F . Gambia: evaluation of the mobile health care service in West Kiang district. World Health Stats Q 1995; 48: 18–22.
Fox-Rushby JA . The Gambia: cost and effectiveness of a mobile maternal health care service, West Kiang. World Health Stats Q 1995; 48: 23–27.
Fullerton JT, Killian R, Gass PM . Outcomes of a community- and home-based intervention for safe motherhood and newborn care. Health Care Women Inter 2005; 26: 561–576.
Haider R, Ashworth A, Kabir I, Huttly SR . Effect of community-based peer counsellors on exclusive breastfeeding practices in Dhaka, Bangladesh: a randomised controlled trial. Lancet 2000; 356: 1643–1647.
Haider R, Kabir I, Huttly SR, Ashworth A . Training peer counselors to promote and support exclusive breastfeeding in Bangladesh. J Hum Lact 2002; 18: 7–12.
Hill AG, MacLeod WB, Joof D, Gomez P, Walraven G . Decline of mortality in children in rural Gambia: the influence of village-level primary health care. Trop Med Inter Health 2000; 5: 107–118.
Jakobsen MS, Sodemann M, Biai S, Nielsen J, Aaby P . Promotion of exclusive breastfeeding is not likely to be cost effective in West Africa. A randomized intervention study from Guinea-Bissau. Acta Paediatr 2008; 97: 68–75.
Jokhio AH, Winter HR, Cheng KK . An intervention involving traditional birth attendants and perinatal and maternal mortality in Pakistan. N Engl J Med 2005; 352: 2091–2099.
Kielmann AA, Taylor CE, Parker RL . The Narangwal Nutrition Study: a summary review. Am J Clin Nutr 1978; 31: 2040–2057.
Kwast BE . Building a community-based maternity program. Int J Gynaecol Obstet 1995; 48 (Suppl): S67–S82.
Leite AJ, Puccini RF, Atalah AN, Alves Da Cunha AL, Machado MT . Effectiveness of home-based peer counselling to promote breastfeeding in the northeast of Brazil: a randomized clinical trial. Acta Paediatr 2005; 94: 741–746.
Manandhar DS, Osrin D, Shrestha BP, Mesko N, Morrison J, Tumbahangphe KM et al. Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster-randomised controlled trial. Lancet 2004; 364: 970–979.
Mbonye AK, Bygbjerg I, Magnussen P . Intermittent preventive treatment of malaria in pregnancy: a community-based delivery system and its effect on parasitemia, anemia and low birth weight in Uganda. Int J Infect Dis 2008; 12: 22–29.
McPherson RA, Khadka N, Moore JM, Sharma M . Are birth-preparedness programmes effective? Results from a field trial in Siraha district, Nepal. J Health Popul Nutr 2006; 24: 479–488.
Meegan ME, Conroy RM, Lengeny SO, Renhault K, Nyangole J . Effect on neonatal tetanus mortality after a culturally-based health promotion programme. Lancet 2001; 358: 640–641.
Mehnaz A, Billoo AG, Yasmeen T, Nankani K . Detection and management of pneumonia by community health workers—a community intervention study in Rehri village, Pakistan. J Pak Med Assoc 1997; 47: 42–45.
Mercer A, Khan MH, Daulatuzzaman M, Reid J . Effectiveness of an NGO primary health care programme in rural Bangladesh: evidence from the management information system. Health Policy Plan 2004; 19: 187–198.
Morrison J, Tamang S, Mesko N, Osrin D, Shrestha B, Manandhar M et al. Women's health groups to improve perinatal care in rural Nepal. BMC Pregnancy Childbirth 2005; 5: 6.
Morrow AL, Guerrero ML, Shults J, Calva JJ, Lutter C, Bravo J et al. Efficacy of home-based peer counselling to promote exclusive breastfeeding: a randomised controlled trial. Lancet 1999; 353: 1226–1231.
Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, Shrestha S et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet 2006; 367: 910–918.
Nankunda J, Tumwine JK, Soltvedt A, Semiyaga N, Ndeezi G, Tylleskar T . Community based peer counsellors for support of exclusive breastfeeding: experiences from rural Uganda. Int Breastfeed J 2006; 1: 19.
O'Rourke K, Howard-Grabman L, Seoane G . Impact of community organization of women on perinatal outcomes in rural Bolivia. Rev Panam Salud Publica 1998; 3: 9–14.
Pence BW, Nyarko P, Phillips JF, Debpuur C . The effect of community nurses and health volunteers on child mortality: the Navrongo Community Health and Family Planning Project. Scand J Pub Health 2007; 35: 599–608.
Perry HB, Shanklin DS, Schroeder DG . Impact of a community-based comprehensive primary healthcare programme on infant and child mortality in Bolivia. J Health Popul Nutr 2003; 21: 383–395.
Phillips JF, Bawah AA, Binka FN . Accelerating reproductive and child health programme impact with community-based services: the Navrongo experiment in Ghana. Bull World Health Organ 2006; 84: 949–955.
Saleem S, Reza T, McClure EM, Pasha O, Moss N, Rouse DJ et al. Chlorhexidine vaginal and neonatal wipes in home births in Pakistan: a randomized controlled trial. Obstet Gynecol 2007; 110: 977–985.
Shah U, Pratinidhi AK, Bhatlawande PV . Perinatal mortality in rural India: intervention through primary health care. II Neonatal mortality. J Epidemiol Community Health 1984; 38: 138–142.
Sibley L, Buffington ST, Beck D, Armbruster D . Home based life saving skills: promoting safe motherhood through innovative community-based interventions. J Midwifery Women's Health 2001; 46: 258–266.
Sibley L, Buffington ST, Haileyesus D . The American College of Nurse-Midwives' home-based lifesaving skills program: a review of the Ethiopia field test. J Midwifery Women's Health 2004; 49: 320–328.
Sibley L, Buffington ST, Tedessa L Sr, McNatt K . Home-based life saving skills in Ethiopia: an update on the second phase of field testing. J Midwifery Women's Health 2006; 51: 284–291.
Sloan NL, Ahmed S, Mitra SN, Choudhury N, Chowdhury M, Rob U et al. Community-based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics 2008; 121 (5): e1047–e1059.
Taha TE, Biggar RJ, Broadhead RL, Mtimavalye LA, Justesen AB, Liomba GN et al. Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. BMJ 1997; 315: 216–219, discussion 220.
Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC et al. Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics 2007; 119: e330–e340.
Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD . Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet 1999; 354: 1955–1961.
Bang AT, Reddy HM, Deshmukh MD, Baitule SB, Bang RA . Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli field trial: effect of home-based neonatal care. J Perinatol 2005; 25 (Suppl 1): S92–107.
Bang AT, Bang RA, Baitule SB, Reddy HM, Deshmukh MD . Management of birth asphyxia in home deliveries in rural Gadchiroli: the effect of two types of birth attendants and of resuscitating with mouth-to-mouth, tube-mask or bag-mask. J Perinatol 2005; 25 (Suppl 1): S82–S91.
Bang AT, Baitule SB, Reddy HM, Deshmukh MD, Bang RA . Low birth weight and preterm neonates: can they be managed at home by mother and a trained village health worker? J Perinatol 2005; 25 (Suppl 1): S72–S81.
Bang AT, Bang RA, Stoll BJ, Baitule SB, Reddy HM, Deshmukh MD . Is home-based diagnosis and treatment of neonatal sepsis feasible and effective? Seven years of intervention in the Gadchiroli field trial (1996 to 2003). J Perinatol 2005; 25 (Suppl 1): S62–S71.
Bang AT, Bang RA, Reddy HM, Deshmukh MD, Baitule SB . Reduced incidence of neonatal morbidities: effect of home-based neonatal care in rural Gadchiroli, India. J Perinatol 2005; 25 (Suppl 1): S51–S61.
Bang AT, Bang RA . Background of the field trial of home-based neonatal care in Gadchiroli, India. J Perinatol 2005; 25 (Suppl 1): S3–10.
Bang AT, Bang RA, Reddy HM, Deshmukh MD . Methods and the baseline situation in the field trial of home-based neonatal care in Gadchiroli, India. J Perinatol 2005; 25 (Suppl 1): S11–S17.
Bang AT, Bang RA, Reddy HM . Home-based neonatal care: summary and applications of the field trial in rural Gadchiroli, India (1993 to 2003). J Perinatol 2005; 25 (Suppl 1): S108–S122.
Ahmed S, Mitra SN, Chowdhury AM, Camacho LL, Winikoff B, Sloan NL . Community Kangaroo Mother Care: implementation and potential for neonatal survival and health in very low-income settings. J Perinatol 2011; 31 (5): 361–367.
Arifeen SE, Hoque DM, Akter T, Rahman M, Hoque ME, Begum K et al. Effect of the integrated management of childhood illness strategy on childhood mortality and nutrition in a rural area in Bangladesh: a cluster randomised trial. Lancet 2009; 374 (9687): 393–403.
Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet 2012; 379 (9820): 1022–1028.
Awasthi S, Srivastava NM, Agarwal GG, Pant S, Ahluwalia TP . Effect of behaviour change communication on qualified medical care-seeking for sick neonates among urban poor in Lucknow, northern India: a before and after intervention study. Trop Med Int Health 2009; 14 (10): 1199–1209.
Azad K, Barnett S, Banerjee B, Shaha S, Khan K, Rego AR et al. Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomised controlled trial. Lancet 2010; 375 (9721): 1193–1202.
Gill CJ, Phiri-Mazala G, Guerina NG, Kasimba J, Mulenga C, MacLeod WB et al. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomised controlled study. BMJ 2011; 342: d346.
Hodgins S, Thapa K, Khanal L, Aryal S, Suvedi BK, Baidya U et al. Chlorhexidine gel versus aqueous for preventive use on umbilical stump: a randomized non inferiority trial. Pediatr Infect Dis J 2010; 29 (11): 999–1003.
Katz KS, Jarrett MH, El-Mohandes AA, Schneider S, McNeely-Johnson D, Kiely M . Effectiveness of a combined home visiting and group intervention for low income African American mothers: the pride in parenting program. Matern Child Health J 2011; 15 (Suppl 1): S75–S84.
Lee E, Mitchell-Herzfeld SD, Lowenfels AA, Greene R, Dorabawila V, DuMont KA . Reducing low birth weight through home visitation: a randomized controlled trial. Am J Prev Med 2009; 36 (2): 154–160.
Lewycka S, Mwansambo C, Kazembe P, Phiri T, Mganga A, Rosato M et al. A cluster randomised controlled trial of the community effectiveness of two interventions in rural Malawi to improve health care and to reduce maternal, newborn and infant mortality. Trials 2010; 11: 88.
Mann V, Fazzio I, King R, Walker P, dos Santos A, de Sa JC et al. The EPICS trial: enabling parents to increase child survival through the introduction of community-based health interventions in rural Guinea Bissau. BMC Public Health 2009; 9: 279.
Matendo R, Engmann C, Ditekemena J, Gado J, Tshefu A, Kinoshita R et al. Reduced perinatal mortality following enhanced training of birth attendants in the Democratic Republic of Congo: a time-dependent effect. BMC Med 2011; 9: 93.
Mullany LC, El Arifeen S, Winch PJ, Shah R, Mannan I, Rahman SM et al. Impact of 4.0% chlorhexidine cleansing of the umbilical cord on mortality and omphalitis among newborns of Sylhet, Bangladesh: design of a community-based cluster randomized trial. BMC Pediatr 2009; 9: 67.
Odendaal W, van Niekerk A, Jordaan E, Seedat M . The impact of a home visitation programme on household hazards associated with unintentional childhood injuries: a randomised controlled trial. Accid Anal Prev 2009; 41 (1): 183–190.
Pasha O, Goldenberg RL, McClure EM, Saleem S, Goudar SS, Althabe F et al. Communities, birth attendants and health facilities: a continuum of emergency maternal and newborn care (the Global Network's EmONC trial). BMC Pregnancy Childbirth 2010; 10: 82.
Soofi S, Cousens S, Imdad A, Bhutto N, Ali N, Bhutta ZA . Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community-based, cluster-randomised trial. Lancet 2012; 379 (9820): 1029–1036.
Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S et al. Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet 2010; 375 (9721): 1182–1192.
Wallin L, Malqvist M, Nga NT, Eriksson L, Persson LA, Hoa DP et al. Implementing knowledge into practice for improved neonatal survival; a cluster-randomised, community-based trial in Quang Ninh province, Vietnam. BMC Health Serv Res 2011; 11: 239.
Wu Z, Viisainen K, Wang Y, Hemminki E . Evaluation of a community-based randomized controlled prenatal care trial in rural China. BMC Health Serv Res 2011; 11: 92.
Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet 2008; 371: 1936–1944.
Bhutta ZA, Memon ZA, Sooi S, Salat MS, Cousens S, Martines J . Implementing community-based perinatal care: results from a pilot study in rural Pakistan. Bull World Health Organ 2008; 86: 452–459.
Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S et al. Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. Lancet 2008; 372: 1151–1162.
Baqui AH, Williams EK, Rosecrans AM, Agrawal PK, Ahmed S, Darmstadt GL et al. Impact of an integrated nutrition and health programme on neonatal mortality in rural northern India. Bull World Health Organ 2008; 86: 796–804.
Baqui AH, Ahmed S, El Arifeen S, Darmstadt GL, Rosecrans AM, Mannan I et al. Effect of timing of first postnatal care home visit on neonatal mortality in Bangladesh: an observational cohort study. BMJ 2009; 339: b2826.
Baqui AH, Arifeen SE, Williams EK, Ahmed S, Mannan I, Rahman SM et al. Effectiveness of home-based management of newborn infections by community health workers in rural Bangladesh. Pediatr Infect Dis J 2009; 28 (4): 304–310.
Bhandari N, Mazumder S, Taneja S, Sommerfelt H, Strand TA . Effect of implementation of Integrated Management of Neonatal and Childhood Illness (IMNCI) programme on neonatal and infant mortality: cluster randomised controlled trial. BMJ 2012; 344: e1634.
Bhutta ZA, Soofi S, Cousens S, Mohammad S, Memon ZA, Ali I et al. Improvement of perinatal and newborn care in rural Pakistan through community-based strategies: a cluster-randomised effectiveness trial. Lancet 2011; 377 (9763): 403–412.
Darmstadt GL, Choi Y, Arifeen SE, Bari S, Rahman SM, Mannan I et al. Evaluation of a cluster-randomized controlled trial of a package of community-based maternal and newborn interventions in Mirzapur, Bangladesh. PloS One 2010; 5 (3): e9696.
Darmstadt GL, El Arifeen S, Choi Y, Bari S, Rahman SM, Mannan I et al. Household surveillance of severe neonatal illness by community health workers in Mirzapur, Bangladesh: coverage and compliance with referral. Health Policy Plan 2010; 25 (2): 112–124.
Carlo WA, Goudar SS, Jehan I, Chomba E, Tshefu A, Garces A et al. Newborn-care training and perinatal mortality in developing countries. New Engl J Med 2010; 362 (7): 614–623.
Acknowledgements
Professor Clive Osmond, MRC Epidemiology Resource Centre, Southampton, UK, helped with statistical analysis in relation to the calculation of cluster-adjusted RRs. This review was funded by the Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland.
Author contributions
SG prepared the protocol, applied the search strategy and retrieved articles. HPSS finalized the protocol and search strategy. Both authors extracted data, performed the statistical analysis and contributed to the drafting of the final version of the paper, and will act as joint guarantors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Gogia, S., Sachdev, H. Home-based neonatal care by community health workers for preventing mortality in neonates in low- and middle-income countries: a systematic review. J Perinatol 36 (Suppl 1), S55–S73 (2016). https://doi.org/10.1038/jp.2016.33
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.33
This article is cited by
-
Interventions addressing maternal and child health among the urban poor and homeless: an overview of systematic reviews
BMC Public Health (2023)
-
Impact of a community-based intervention package delivered through community health workers on post-partum care practices: a cluster randomized controlled trial
Journal of Public Health (2023)
-
The effects of completion of continuum of care in maternal health services on adverse birth outcomes in Northwestern Ethiopia: a prospective follow-up study
Reproductive Health (2022)
-
Community Health Worker Impact on Knowledge, Antenatal Care, And Birth Outcomes: A Systematic Review
Maternal and Child Health Journal (2022)
-
“I had to change my attitude”: narratives of most significant change explore the experience of universal home visits to pregnant women and their spouses in Bauchi State, Nigeria
Archives of Public Health (2021)