Abstract
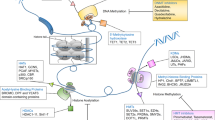
Epigenetic changes have been identified in recent years as important factors in the pathogenesis of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Histone deacetylase inhibitors (HDACIs) regulate the acetylation of histones as well as other non-histone protein targets. Treatment with HDACIs results in chromatin remodeling that permits re-expression of silenced tumor suppressor genes in cancer cells, which, in turn, can potentially result in cellular differentiation, inhibition of proliferation and/or apoptosis. Several classes of HDACIs are currently under development for the treatment of patients with MDS and AML. Although modest clinical activity has been reported with the use of HDACIs as single-agent therapy, marked responses have been observed in selected subsets of patients. More importantly, HDACIs appear to be synergistic in vitro and improve response rates in vivo when combined with other agents, such as hypomethylating agents. Furthermore, HDACIs are also being investigated in combination with non-epigenetic therapies. This article synthesizes the most recent results reported with HDACIs in clinical trials conducted in patients with MDS and other myeloid malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Heaney ML, Golde DW . Myelodysplasia. N Engl J Med 1999; 340: 1649–1660.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn. IARC Press: Lyon, 2008.
Ravandi F, Burnett AK, Agura ED, Kantarjian HM . Progress in the treatment of acute myeloid leukemia. Cancer 2007; 110: 1900–1910.
Estey E, Thall P, Beran M, Kantarjian H, Pierce S, Keating M . Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood 1997; 90: 2969–2977.
Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010, (doi: 10.1182/blood-2010-03-276485).
Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood 2007; 109: 1395–1400.
Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006; 106: 1090–1098.
Griffiths EA, Gore SD . DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol 2008; 45: 23–30.
Minucci S, Pelicci PG . Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51.
Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA 1998; 95: 3003–3007.
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK . Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001; 1: 194–202.
Bhalla KN . Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol 2005; 23: 3971–3993.
Felsenfeld G, Groudine M . Controlling the double helix. Nature 2003; 421: 448–453.
Rice JC, Allis CD . Code of silence. Nature 2001; 414: 258–261.
Yang XJ, Seto E . HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007; 26: 5310–5318.
Brandl A, Heinzel T, Kramer OH . Histone deacetylases: salesmen and customers in the post-translational modification market. Biol Cell 2009; 101: 193–205.
Gregoretti I, Lee Y-M, Goodson HV . Molecular evolution of the histone deacetylase family: functional implication of phylogenetic analysis. J Mol Biol 2004; 338: 17–31.
Xu WS, Parmigiani RB, Marks PA . Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 2007; 26: 5541–5552.
Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 2005; 37: 391–400.
Nakagawa M, Oda Y, Eguchi T, Aishima S, Yao T, Hosoi F et al. Expression profile of class I histone deacetylases in human cancer tissues. Oncol Rep 2007; 18: 769–774.
Lin RJ, Nagy L, Inoue S, Shao W, Miller Jr WH, Evans RM . Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 1998; 391: 811–814.
Gelmetti V, Zhang J, Fanelli M, Minucci S, Pelicci PG, Lazar MA . Aberrant recruitment of the nuclear receptor corepressor–histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol Cell Biol 1998; 18: 7185–7191.
Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M et al. Fusion proteins of the retinoic acid receptor–alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 1998; 391: 815–818.
Martens JH, Brinkman AB, Simmer F, Francoijs KJ, Nebbioso A, Ferrara F et al. PML-RARalpha/RXR alters the epigenetic landscape in acute promyelocytic leukemia. Cancer Cell 2010; 17: 173–185.
Altucci L, Gronemeyer H . The promise of retinoids to fight against cancer. Nat Rev Cancer 2001; 1: 181–193.
Chambers AE, Banerjee S, Chaplin T, Dunne J, Debernardi S, Joel SP et al. Histone acetylation-mediated regulation of genes in leukaemic cells. Eur J Cancer 2003; 39: 1165–1175.
Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci USA 2005; 102: 3697–3702.
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA . Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA 2004; 101: 1241–1246.
Archer SY, Meng S, Shei A, Hodin RA . p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA 1998; 95: 6791–6796.
Huang L, Sowa Y, Sakai T, Pardee AB . Activation of the p21WAF1/CIP1 promoter independent of p53 by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through the Sp1 sites. Oncogene 2000; 19: 5712–5719.
Rosato RR, Almenara JA, Grant S . The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF11. Cancer Res 2003; 63: 3637–3645.
Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med 2005; 11: 77–84.
Davis T, Kennedy C, Chiew YE, Clarke CL, deFazio A . Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin Cancer Res 2000; 6: 4334–4342.
Robbins AR, Jablonski SA, Yen TJ, Yoda K, Robey R, Bates SE et al. Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin. Cell Cycle 2005; 4: 717–726.
Cimini D, Mattiuzzo M, Torosantucci L, Degrassi F . Histone hyperacetylation in mitosis prevents sister chromatid separation and produces chromosome segregation defects. Mol Biol Cell 2003; 14: 3821–3833.
Qiu L, Burgess A, Fairlie DP, Leonard H, Parsons PG, Gabrielli BG . Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell 2000; 11: 2069–2083.
Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med 2005; 11: 71–76.
Zhang XD, Gillespie SK, Borrow JM, Hersey P . The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther 2004; 3: 425–435.
Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q . Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci USA 2005; 102: 16090–16095.
Xu W, Ngo L, Perez G, Dokmanovic M, Marks PA . Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitor. Proc Natl Acad Sci USA 2006; 103: 15540–15545.
Rosato RR, Maggio SC, Almenara JA, Payne SG, Atadja P, Spiegel S et al. The histone deacetylase inhibitor LAQ824 induces human leukemia cell death through a process involving XIAP down-regulation, oxidative injury, and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol 2006; 69: 216–225.
Rosato RR, Almenara JA, Dai Y, Grant S . Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther 2003; 2: 1273–1284.
Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA 2001; 98: 10833–10838.
Fiskus W, Rao R, Fernandez P, Herger B, Yang Y, Chen J et al. Molecular and biologic characterization and drug sensitivity of pan-histone deacetylase inhibitor-resistant acute myeloid leukemia cells. Blood 2008; 112: 2896–2905.
Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE . Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther 2006; 5: 1967–1974.
Ozaki K, Kishikawa F, Tanaka M, Sakamoto T, Tanimura S, Kohno M . Histone deacetylase inhibitors enhance the chemosensitivity of tumor cells with cross-resistance to a wide range of DNA-damaging drugs. Cancer Sci 2008; 99: 376–384.
Zhang Y, Adachi M, Zou H, Hareyama M, Imai K, Shinomura Y . Histone deacetylase inhibitors enhance phosphorylation of histone H2AX after ionizing radiation. Int J Radiat Oncol Biol Phys 2006; 65: 859–866.
Gaymes TJ, Padua RA, Pla M, Orr S, Omidvar N, Chomienne C et al. Histone deacetylase inhibitors (HDI) cause DNA damage in leukemia cells: a mechanism for leukemia-specific HDI-dependent apoptosis? Mol Cancer Res 2006; 4: 563–573.
Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, Hoshino K, Quintas-Cardama A, Richon VM et al. Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood 2006; 108: 1174–1182.
Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther 2008; 7: 3285–3297.
Chen CS, Wang YC, Yang HC, Huang PH, Kulp SK, Yang CC et al. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res 2007; 67: 5318–5327.
Hu Y, Lu W, Chen G, Zhang H, Jia Y, Wei Y et al. Overcoming resistance to histone deacetylase inhibitors in human leukemia with the redox modulating compound {beta}-phenylethyl isothiocyanate. Blood 2010; 116: 2732–2741.
Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci USA 2005; 102: 673–678.
Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 2008; 111: 1060–1066.
Shao Y, Gao Z, Marks PA, Jiang X . Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA 2004; 101: 18030–18035.
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE . Cell death. N Engl J Med 2009; 361: 1570–1583.
Wei Y, Kadia T, Tong W, Zhang M, Jia Y, Yang H et al. The combination of a histone deacetylase inhibitor with the Bcl-2 homology domain-3 mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Clin Cancer Res 2010; 16: 3923–3932.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 2005; 280: 26729–26734.
Aoyagi S, Archer TK . Modulating molecular chaperone Hsp90 functions through reversible acetylation. Trends Cell Biol 2005; 15: 565–567.
Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB . Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 1999; 21: 103–107.
Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. Embo J 2001; 20: 6969–6978.
Cimino G, Lo-Coco F, Fenu S, Travaglini L, Finolezzi E, Mancini M et al. Sequential valproic acid/all-trans retinoic acid treatment reprograms differentiation in refractory and high-risk acute myeloid leukemia. Cancer Res 2006; 66: 8903–8911.
Trus MR, Yang L, Suarez Saiz F, Bordeleau L, Jurisica I, Minden MD . The histone deacetylase inhibitor valproic acid alters sensitivity towards all trans retinoic acid in acute myeloblastic leukemia cells. Leukemia 2005; 19: 1161–1168.
Kuendgen A, Strupp C, Aivado M, Bernhardt A, Hildebrandt B, Haas R et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood 2004; 104: 1266–1269.
Kuendgen A, Knipp S, Fox F, Strupp C, Hildebrandt B, Steidl C et al. Results of a phase 2 study of valproic acid alone or in combination with all-trans retinoic acid in 75 patients with myelodysplastic syndrome and relapsed or refractory acute myeloid leukemia. Ann Hematol 2005; 84 (Suppl 1): 61–66.
Bug G, Ritter M, Wassmann B, Schoch C, Heinzel T, Schwarz K et al. Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia. Cancer 2005; 104: 2717–2725.
Kuendgen A, Schmid M, Schlenk R, Knipp S, Hildebrandt B, Steidl C et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer 2006; 106: 112–119.
Raffoux E, Chaibi P, Dombret H, Degos L . Valproic acid and all-trans retinoic acid for the treatment of elderly patients with acute myeloid leukemia. Haematologica 2005; 90: 986–988.
Pilatrino C, Cilloni D, Messa E, Morotti A, Giugliano E, Pautasso M et al. Increase in platelet count in older, poor-risk patients with acute myeloid leukemia or myelodysplastic syndrome treated with valproic acid and all-trans retinoic acid. Cancer 2005; 104: 101–109.
Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood 2007; 110: 2302–2308.
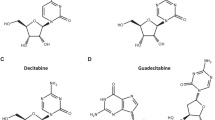
Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood 2006; 108: 3271–3279.
Issa J-P, Castoro R, Ravandi-Kashani F, Faderl S, Huang X, Estey E et al. Randomized phase II study of combined epigenetic therapy: decitabine vs decitabine and valproic acid in MDS and AML. Blood 2008; 112 (abstract 228).
Marks PA, Miller T, Richon VM . Histone deacetylases. Curr Opin Pharmacol 2003; 3: 344–351.
Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R . FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007; 12: 1247–1252.
Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res 2003; 9 (Part 1): 3578–3588.
Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 2005; 23: 3923–3931.
Schaefer EW, Loaiza-Bonilla A, Juckett M, DiPersio JF, Roy V, Slack J et al. A phase 2 study of vorinostat in acute myeloid leukemia. Haematologica 2009; 94: 1375–1382.
Silverman LR, Verma A, Odchimar-Reissig R, LeBlanc A, Najfeld V, Gabrilove J et al. A phase I trial of the epigenetic modulators vorinostat, in combination with azacitidine (azaC) in patients with the myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML): A Study of the New York Cancer Consortium. Blood 2008; 112, (abstract 3656).
Kirschbaum M, Gojo I, Goldberg SL, Kujawski L, Atallah E, Marks P et al. Vorinostat in combination with decitabine for the treatment of relapsed or newly diagnosed acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS): a phase I, Dose-Escalation Study. Blood 2009; 114 (abstract 2089).
Ravandi F, Faderl S, Thomas D, Burger J, Koller C, Garcia-Manero G et al. Phase I study of sSuberoylanilide hydroxamic acid (SAHA) and ecitabine in patients with relapsed, refractory or poor prognosis leukemia. Blood 2007; 110 (abstract 897).
Shiozawa K, Nakanishi T, Tan M, Fang HB, Wang WC, Edelman MJ et al. Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin Cancer Res 2009; 15: 1698–1707.
Garcia-Manero G, Tambaro FP, Bekele BN, Jabbour E, Ravandi F, Yang H et al. Phase II study of vorinostat in combination with idarubicin (Ida) and cytarabine (ara-C) as front line therapy in acute myelogenous leukemia (AML) or higher risk myelodysplastic syndrome (MDS). Blood 2009; 114 (abstract 1055).
Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget MC, Kalita A et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther 2008; 7: 759–768.
Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood 2008; 112: 981–989.
Garcia-Manero G, Yang AS, Klimek V, Cortes J, Ravandi F, Newsome WM et al. Phase I/II Study of MGCD0103, an oral isotype-selective histone deacetylase (HDAC) inhibitor, in combination with 5-azacitidine in higher-risk myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML). Blood 2007; 110 (abstract 444).
Gojo I, Jiemjit A, Trepel JB, Sparreboom A, Figg WD, Rollins S et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood 2007; 109: 2781–2790.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Gore SD, Jiemjit A, Silverman LB, Aucott T, Baylin S, Carraway H et al. Combined methyltransferase/histone deacetylase inhibition with 5-Azacitidine and MS-275 in patients with MDS, CMMoL and AML: clinical response, histone acetylation and DNA damage. Blood 2006; 108 (abstract 517).
Fandy TE, Herman JG, Kerns P, Jiemjit A, Sugar EA, Choi SH et al. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood 2009; 114: 2764–2773.
Ellis L, Pan Y, Smyth GK, George DJ, McCormack C, Williams-Truax R et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res 2008; 14: 4500–4510.
Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 2006; 12: 4628–4635.
Furumai R, Matsuyama A, Kobashi N, Lee KH, Nishiyama M, Nakajima H et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res 2002; 62: 4916–4921.
Itoh Y, Suzuki T, Miyata N . Isoform-selective histone deacetylase inhibitors. Curr Pharm Des 2008; 14: 529–544.
Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010; 28: 4485–4491.
Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 2005; 105: 959–967.
Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, Watkins CJ et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther 2003; 2: 721–728.
Gimsing P, Wu F, Qian X, Jeffers M, Knudsen L, Sehested M et al. Activity of the histone deacetylase (HDAC) inhibitor PXD101 in preclinical studies and in a phase I study in patients with advanced haematological tumors. Blood 2005; 106 (abstract 3337).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
AQC and FPSS designed the research and wrote the manuscript. GGM supervised the research and approved the manuscript.
Rights and permissions
About this article
Cite this article
Quintás-Cardama, A., Santos, F. & Garcia-Manero, G. Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia 25, 226–235 (2011). https://doi.org/10.1038/leu.2010.276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.276
Keywords
This article is cited by
-
The NCOR-HDAC3 co-repressive complex modulates the leukemogenic potential of the transcription factor ERG
Nature Communications (2023)
-
Acute Myeloid Leukemia in Children: Emerging Paradigms in Genetics and New Approaches to Therapy
Current Oncology Reports (2021)
-
Preclinical evaluation of a regimen combining chidamide and ABT-199 in acute myeloid leukemia
Cell Death & Disease (2020)
-
A new insight into base excision repair (BER) in targeted cancer therapy
Genome Instability & Disease (2020)
-
CCCTC-binding factor is essential to the maintenance and quiescence of hematopoietic stem cells in mice
Experimental & Molecular Medicine (2017)