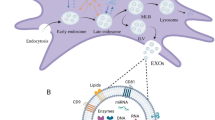
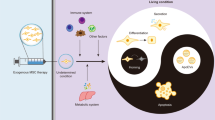
Abstract
Although regenerative medicine is searching for pluripotent stem cells that could be employed for therapy, various types of more differentiated adult stem and progenitor cells are in meantime being employed in clinical trials to regenerate damaged organs (for example, heart, kidney or neural tissues). It is striking that, for a variety of these cells, the currently observed final outcomes of cellular therapies are often similar. This fact and the lack of convincing documentation for donor–recipient chimerism in treated tissues in most of the studies indicates that a mechanism other than transdifferentiation of cells infused systemically into peripheral blood or injected directly into damaged organs may have an important role. In this review, we will discuss the role of (i) growth factors, cytokines, chemokines and bioactive lipids and (ii) microvesicles (MVs) released from cells employed as cellular therapeutics in regenerative medicine. In particular, stem cells are a rich source of these soluble factors and MVs released from their surface may deliver RNA and microRNA into damaged organs. Based on these phenomena, we suggest that paracrine effects make major contributions in most of the currently reported positive results in clinical trials employing adult stem cells. We will also present possibilities for how these paracrine mechanisms could be exploited in regenerative medicine to achieve better therapeutic outcomes. This approach may yield critical improvements in current cell therapies before true pluripotent stem cells isolated in sufficient quantities from adult tissues and successfully expanded ex vivo will be employed in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001; 97: 3075–3085.
Janowska-Wieczorek A, Majka M, Ratajczak J, Ratajczak MZ . Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells 2001; 19: 99–107.
Sahoo S, Klychko E, Thorne T, Misener S, Schults KM, Millay M et al. Exosomesfrom human CD34+ stem cells mediate their proangiopoietic paracrine activity. Cir Res 2011; 109: 724–728.
Lataillade JJ, Clay D, Bourin P, Hérodin F, Dupuy C, Jasmin C et al. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: evidence for an autocrine/paracrine mechanism. Blood 2002; 99: 1117–1129.
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ . Membrane-derived microvesicles (MV): important and underappreciated mediators of cell to cell communication. Leukemia 2006; 20: 1487–1495.
Quesenberry PJ, Dooner MS, Aliotta JM . Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp Hematol 2010; 38: 581–592.
Camussi G, Deregibus MC, Tetta C . Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens 2010; 19: 7–12.
Beaudoin AR, Grondin G . Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Bioch et Biophys Acta 1991; 1071: 203–219.
Fevrier B, Raposo G . Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004; 16: 415–421.
Greenwalt TJ . The how and why of exocytic vesicles. Transfusion 2006; 46: 143–152.
Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM . Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005; 20: 22–27.
Barry OP, FitzGerald GA . Mechanisms of cellular activation by platelet microparticles. Thrombosis Haemostasis 1999; 82: 794–800.
Barry OP, Pratico D, Savani RC, FitzGerald GA . Modulation of monocyte-endothelial cell interaction by platelet microparticles. J Clin Invest 1998; 102: 136–144.
VanWijk MJ, VanBavel E, Sturk A, Nieuwland R . Microparticles in cardiovascular diseases. Cardiovasc Res 2003; 59: 277–287.
Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS . New horizons in the analysis of circulating cell-derived microparticles. Keio J Med 2004; 53: 210–230.
Greco V, Hannus M, Eaton S . Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 2001; 106: 633–645.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006; 20: 847–856.
Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dialysis Transplant 2011; 26: 1474–1483.
Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J 2009; 30: 1313–1321.
Howe AJ, Shand JA, Menown IB . Advances in cardiology: clinical trial update. Future Cardiol 2011; 7: 299–310.
Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M . Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia 2011, e-pub ahead of print 26 July 2011; doi: 10.1038/leu.2011.184.
Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W et al. Hunt for pluripotent stem cell -- regenerative medicine search for almighty cell. J Autoimmun 2008; 30: 151–162.
Borlongan CV . Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all!. Leukemia 2011; 25: 1674–1686.
Staal FJ, Baum C, Cowan C, Dzierzak E, Hacein-Bey-Abina S, Karlsson S et al. Stem cell self-renewal: lessons from bone marrow, gut and iPS toward clinical applications. Leukemia 2011; 25: 1095–1102.
Di Campli C, Piscaglia AC, Pierelli L, Rutella S, Bonanno G, Alison MR et al. A human umbilical cord stem cell rescue therapy in a murine model of toxic liver injury. Dig Liver Dis 2004; 36: 603–613.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–705.
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000; 6: 1229–1234.
Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR . Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000; 290: 1779–1782.
Herzog EL, Chai L, Krause DS . Plasticity of marrow-derived stem cells. Blood 2003; 102: 3483–3493.
Corti S, Locatelli F, Donadoni C, Strazzer S, Salani S, Del Bo R et al. Neuroectodermal and microglial differentiation of bone marrow cells in the mouse spinal cord and sensory ganglia. J Neurosci Res 2002; 70: 721–733.
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N et al. Bone marrow as a potential source of hepatic oval cells. Science 1999; 284: 1168–1170.
Wagers AJ, Sherwood RI, Christensen JL, Weissman IL . Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002; 297: 2256–2259.
Murry CE, Soonpaa MH, Reinecke H et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004; 428: 664–668.
Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD . Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science 2002; 297: 1299.
Kucia M, Ratajczak J, Ratajczak MZ . Are bone marrow stem cells plastic or heterogenous – that is the question. Exp Hematol 2005; 33: 613–623.
Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS . Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004; 305: 90–93.
Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006; 20: 857–869.
Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ . Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tiss Res 2008; 331: 125–134.
Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M et al. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia 2010; 24: 1450–1461.
Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia 2009; 23: 2042–2051.
Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 2011; 183: 215–225.
Myers TJ, Granero-Molto F, Longobardi L, Li T, Yan Y, Spagnoli A . Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther 2010; 10: 1663–1679.
Spees JL, Olson SD, Whitney MJ, Prockop DJ . Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 2006; 103: 1283–1288.
Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010; 5: e11803.
Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia 2010; 24: 976–985.
Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ et al. A novel perspective on stem cell homing and mobilization – review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 2011, e-pub ahead of print 2 September 2011; doi: 10.1038/leu.2011.242.
Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 2007; 25: 2245–2256.
Ratajczak J, Kijowski J, Majka M, Jankowski K, Reca R, Ratajczak MZ . Biological significance of the different erythropoietic factors secreted by normal human early erythroid cells. Leukemia Lymphoma 2003; 44: 767–774.
Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 2010; 14: 1605–1618.
Ceradini DJ, Gurtner GC . Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 2005; 15: 57–63.
Acknowledgements
This work was supported by NIH Grant R01 DK074720, the Stella and Henry Endowment, and the European Union structural funds (Innovative Economy Operational Program POIG.01.01.02-00-109/09-00) to MZR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ratajczak, M., Kucia, M., Jadczyk, T. et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies?. Leukemia 26, 1166–1173 (2012). https://doi.org/10.1038/leu.2011.389
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.389
Keywords
This article is cited by
-
Leukemogenesis occurs in a microenvironment enriched by extracellular microvesicles/exosomes: recent discoveries and questions to be answered
Leukemia (2024)
-
Role of exosome-derived miRNAs in diabetic wound angiogenesis
Molecular and Cellular Biochemistry (2023)
-
Secretome of human umbilical cord mesenchymal stem cell maintains skin homeostasis by regulating multiple skin physiological function
Cell and Tissue Research (2023)
-
Conditioned Medium – Is it an Undervalued Lab Waste with the Potential for Osteoarthritis Management?
Stem Cell Reviews and Reports (2023)
-
Strategies of cell and cell-free therapies for periodontal regeneration: the state of the art
Stem Cell Research & Therapy (2022)