Abstract
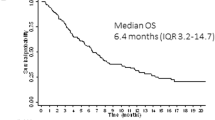
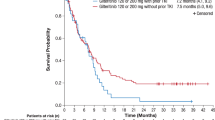
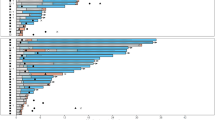
Preliminary evidence suggests that the multikinase inhibitor sorafenib has clinical activity in FLT3-ITD-positive (FLT3-ITD) acute myeloid leukemia (AML). However, the quality and sustainability of achievable remissions and clinical variables that influence the outcome of sorafenib monotherapy are largely undefined. To address these questions, we evaluated sorafenib monotherapy in 65 FLT3-ITD AML patients treated at 23 centers. All but two patients had relapsed or were chemotherapy-refractory after a median of three prior chemotherapy cycles. Twenty-nine patients (45%) had undergone prior allogeneic stem cell transplantation (allo-SCT). The documented best responses were: hematological remission in 24 patients (37%), bone marrow remission in 5 patients (8%), complete remission (with and without normalization of peripheral blood counts) in 15 patients (23%) and molecular remission with undetectable FLT3-ITD mRNA in 10 patients (15%), respectively. Seventeen of the patients without prior allo-SCT (47%) developed sorafenib resistance after a median treatment duration of 136 days (range, 56–270 days). In contrast, allo-SCT patients developed sorafenib resistance less frequently (38%) and significantly later (197 days, range 38–225 days; P=0.03). Sustained remissions were seen exclusively in the allo-SCT cohort. Thus, sorafenib monotherapy has significant activity in FLT3-ITD AML and may synergize with allogeneic immune effects to induce durable remissions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2009; 115: 453–474.
Kindler T, Lipka DB, Fischer T . FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 2010; 116: 5089–5102.
Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 2002; 100: 4372–4380.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG . FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 2002; 99: 310–318.
Grundler R, Miething C, Thiede C, Peschel C, Duyster J . FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood 2005; 105: 4792–4799.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60.
Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood 2006; 108: 3262–3270.
Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 2004; 103: 3669–3676.
Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst 2008; 100: 184–198.
Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia 2010; 24: 1437–1444.
Crump M, Hedley D, Kamel-Reid S, Leber B, Wells R, Brandwein J et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma 2010; 51: 252–260.
Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica 2010; 96: 62–68.
Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M . Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008; 7: 3129–3140.
Scholl S, Spies-Weisshart B, Klink A, Muegge LO, Fricke HJ, Hochhaus A . Secondary resistance to sorafenib in two patients with acute myeloid leukemia (AML) harboring FLT3-ITD mutations. Ann Hematol 2011; 90: 473–475.
Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood 2009; 113: 6567–6571.
Metzelder SK, Wollmer E, Neubauer A, Burchert A . Sorafenib in relapsed and refractory FLT3-ITD positive acute myeloid leukemia: a novel treatment option. Dtsch Med Wochenschr 2010; 135: 1852–1856.
Safaian NN, Czibere A, Bruns I, Fenk R, Reinecke P, Dienst A et al. Sorafenib (Nexavar((R))) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD(+) acute myeloid leukemia. Leuk Res 2009; 33: 348–350.
Lee SH, Paietta E, Racevskis J, Wiernik PH . Complete resolution of leukemia cutis with sorafenib in an acute myeloid leukemia patient with FLT3-ITD mutation. Am J Hematol 2009; 84: 701–702.
Schroeder T, Zohren F, Saure C, Bruns I, Czibere A, Safaian NN et al. Sorafenib treatment in 13 patients with acute myeloid leukemia and activating FLT3 mutations in combination with chemotherapy or as monotherapy. Acta Haematol 2010; 124: 153–159.
Mori M, Sprague J . The successful remission induction by sorafenib and long-term complete remission in a FLT3-ITD-positive patient with a refractory acute erythroid leukemia and abnormal cytogenetics. Leuk Res 2011; 36: e1–e3.
Mohan BP, How GF, Loh Y, Linn YC . Sorafenib monotherapy gives sustainable suppression of FLT3 clone in untreated patients with FLT3-internal tandem duplication positive acute myeloid Leukaemia. Br J Haematol 2012; 157: 131–132.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Rollig C, Brandts C, Shaid S, Hentrich M, Kramer A, Junghanss C et al. Survey and analysis of the efficacy and prescription pattern of sorafenib in patients with acute myeloid leukemia. Leuk Lymphoma 2011; e-pub ahead of print 7 December 2011; doi:10.3109/10428194.2011.637210.
Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 2010; 28: 4339–4345.
Cortes JE, Perl AE, Smith CC, Kovacsovics T, Dombret H, Dohner H et al. A phase II open-label, Ac220 monotherapy efficacy study in patients with refractory/relapsed Flt3-Itd positive acute myeloid leukemia: updated interim results. ASH Annu Meet Abstr 2011; 118: 2576.
Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 1856–1862.
Serve H, Wagner R, Sauerland C, Brunnberg U, Krug U, Schaich M et al. Sorafenib in combination with standard induction and consolidation therapy in elderly AML patients: results from a randomized placebo-controlled phase II trial. ASH Annu Meet Abstr 2010; 116: 333.
Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 2011; 117: 3294–3301.
Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood 2011; 117: 3286–3293.
Winkler J, Rech D, Kallert S, Rech J, Meidenbauer N, Roesler W et al. Sorafenib induces sustained molecular remission in FLT3-ITD positive AML with relapse after second allogeneic stem cell transplantation without exacerbation of acute GVHD: a case report. Leuk Res 2010; 34: e270–e272.
Sora F, Chiusolo P, Metafuni E, Bellesi S, Giammarco S, Laurenti L et al. Sorafenib for refractory FMS-like tyrosine kinase receptor-3 (FLT3/ITD+) acute myeloid leukemia after allogenic stem cell transplantation. Leuk Res 2011; 35: 422–423.
Hess G, Bunjes D, Siegert W, Schwerdtfeger R, Ledderose G, Wassmann B et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol 2005; 23: 7583–7593.
Yokoyama H, Lundqvist A, Su S, Childs R . Toxic effects of sorafenib when given early after allogeneic hematopoietic stem cell transplantation. Blood 2010; 116: 2858–2859.
Sharma M, Ravandi F, Bayraktar UD, Chiattone A, Bashir Q, Giralt S et al. Treatment of FLT3-ITD-positive acute myeloid leukemia relapsing after allogeneic stem cell transplantation with sorafenib. Biol Blood Marrow Transplant 2011; 17: 1874–1877.
Acknowledgements
We are particularly thankful to Michael Lübbert for critically reading the manuscript and Thomas Pabst, Kristina Siegloch, Julian Sprague for contributing their patients. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Klinische Forschergruppe KFO210 TP1 (to AB) and TP3 (to AN), the Transregio SFB 17 (to AB und AN), the Behring Röntgen Foundation TP51-0057 (to AB) by a research grant of the University Medical Center Gießen and Marburg TP 24/2010 (to AB), the LOEWE-consortium ‘Tumor and Inflammation’ (to AB and AN) and the German Carreras Leukemia Foundation AH 06-01 and AR 08-05v (to AN) and R 11-03 (to SS).
SKM treated the patients, performed the research, analyzed the data, compiled the figures and helped in writing the paper. All co-investigators treated patients, collected and provided clinical data. AB treated patients, designed and coordinated the research, analyzed the data and wrote the paper. AN coordinated the research and discussed the data. All authors critically reviewed and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part as abstract at the 51st and 52nd annual meeting of the American Society of Hematology, New Orleans, LA, 2009 and Orlando, FL, 2010.
Rights and permissions
About this article
Cite this article
Metzelder, S., Schroeder, T., Finck, A. et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia 26, 2353–2359 (2012). https://doi.org/10.1038/leu.2012.105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.105
Keywords
This article is cited by
-
Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2022)
-
FLT3-inhibitor therapy for prevention and treatment of relapse after allogeneic hematopoietic cell transplantation
International Journal of Hematology (2022)
-
Sorafenib maintenance after hematopoietic stem cell transplantation improves outcome of FLT3–ITD-mutated acute myeloid leukemia
International Journal of Hematology (2022)
-
FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm
Blood Cancer Journal (2021)
-
Pre-emptive use of Sorafenib combined with DLI post HSCT in AML FLT3+: a single center experience
Bone Marrow Transplantation (2021)