Abstract
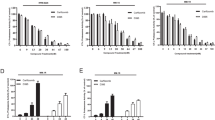
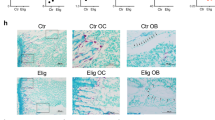
Proteasome inhibitors (PIs), namely bortezomib, have become a cornerstone therapy for multiple myeloma (MM), potently reducing tumor burden and inhibiting pathologic bone destruction. In clinical trials, carfilzomib, a next generation epoxyketone-based irreversible PI, has exhibited potent anti-myeloma efficacy and decreased side effects compared with bortezomib. Carfilzomib and its orally bioavailable analog oprozomib, effectively decreased MM cell viability following continual or transient treatment mimicking in vivo pharmacokinetics. Interactions between myeloma cells and the bone marrow (BM) microenvironment augment the number and activity of bone-resorbing osteoclasts (OCs) while inhibiting bone-forming osteoblasts (OBs), resulting in increased tumor growth and osteolytic lesions. At clinically relevant concentrations, carfilzomib and oprozomib directly inhibited OC formation and bone resorption in vitro, while enhancing osteogenic differentiation and matrix mineralization. Accordingly, carfilzomib and oprozomib increased trabecular bone volume, decreased bone resorption and enhanced bone formation in non-tumor bearing mice. Finally, in mouse models of disseminated MM, the epoxyketone-based PIs decreased murine 5TGM1 and human RPMI-8226 tumor burden and prevented bone loss. These data demonstrate that, in addition to anti-myeloma properties, carfilzomib and oprozomib effectively shift the bone microenvironment from a catabolic to an anabolic state and, similar to bortezomib, may decrease skeletal complications of MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Basak GW, Srivastava AS, Malhotra R, Carrier E . Multiple myeloma bone marrow niche. Curr Pharm Biotechnol 2009; 10: 345–346.
Esteve FR, Roodman GD . Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol 2007; 20: 613–624.
Yaccoby S . Osteoblastogenesis and tumor growth in myeloma. Leuk Lymphoma 2010; 51: 213–220.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Kyle RA, Rajkumar SV . Multiple myeloma. Blood 2008; 111: 2962–2972.
de Bettignies G, Coux O . Proteasome inhibitors: Dozens of molecules and still counting. Biochimie 2010; 92: 1530–1545.
Dick LR, Fleming PE . Building on bortezomib: s-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today 2010; 15: 243–249.
Hideshima T, Anderson KC . Preclinical studies of novel targeted therapies. Hematol Oncol Clin North Am 2007; 21: 1071–1091, viii-ix.
Terpos E, Sezer O, Croucher P, Dimopoulos MA . Myeloma bone disease and proteasome inhibition therapies. Blood 2007; 110: 1098–1104.
Terpos E, Dimopoulos MA, Sezer O, Roodman D, Abildgaard N, Vescio R et al. The use of biochemical markers of bone remodeling in multiple myeloma: a report of the International Myeloma Working Group. Leukemia 2010; 24: 1700–1712.
Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest 2008; 118: 491–504.
Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S . The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol 2009; 84: 6–14.
Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Munoz S et al. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol 2007; 139: 434–438.
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 2007; 110: 334–338.
Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B et al. Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood 2009; 113: 4319–4330.
De Matteo M, Brunetti AE, Maiorano E, Cafforio P, Dammacco F, Silvestris F . Constitutive down-regulation of Osterix in osteoblasts from myeloma patients: in vitro effect of Bortezomib and Lenalidomide. Leuk Res 2010; 34: 243–249.
von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M et al. Bortezomib inhibits human osteoclastogenesis. Leukemia 2007; 21: 2025–2034.
Boissy P, Andersen TL, Lund T, Kupisiewicz K, Plesner T, Delaisse JM . Pulse treatment with the proteasome inhibitor bortezomib inhibits osteoclast resorptive activity in clinically relevant conditions. Leuk Res 2008; 32: 1661–1668.
Orlowski RZ, Kuhn DJ . Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res 2008; 14: 1649–1657.
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 2006; 24: 3113–3120.
Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 2007; 110: 3281–3290.
Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res 2007; 67: 6383–6391.
Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood 2009; 114: 3439–3447.
Singhal SB, DSd Siegel, Martin T, Vij R, Wang M, Jakubowiak AJ et al. Pooled safety analysis from phase (Ph) 1 and 2 studies of carfilzomib (CFZ) in patients with relapsed and/or refractory multiple myeloma (MM). Blood 2010; 116, abstract 1954.
Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood, e-pub ahead of print 3 May 2012; doi:10.1182/blood-2012-03-414359.
Zhou HJ, Aujay MA, Bennett MK, Dajee M, Demo SD, Fang Y et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047). J Med Chem 2009; 52: 3028–3038.
Chauhan D, Singh AV, Aujay M, Kirk CJ, Bandi M, Ciccarelli B et al. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood 2011; 116: 4906–4915.
Papadopoulos KP, Mendelson DS, Tolcher AW, Patnaik A, Burris HA, Rasco DW et al. A phase I, open-label, dose-escalation study of the novel oral proteasome inhibitor (PI) ONX 0912 in patients with advanced refractory or recurrent solid tumors. J Clin Oncol 2011; 29, abstract 3075.
Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest 2003; 111: 1771–1782.
Ang E, Pavlos NJ, Rea SL, Qi M, Chai T, Walsh JP et al. Proteasome inhibitors impair RANKL-induced NF-kappaB activity in osteoclast-like cells via disruption of p62, TRAF6, CYLD, and IkappaBalpha signaling cascades. J Cell Physiol 2009; 220: 450–459.
Carvajal-Vergara X, Tabera S, Montero JC, Esparis-Ogando A, Lopez-Perez R, Mateo G et al. Multifunctional role of Erk5 in multiple myeloma. Blood 2005; 105: 4492–4499.
Garrett IR, Dallas S, Radl J, Mundy GR . A murine model of human myeloma bone disease. Bone 1997; 20: 515–520.
Garcia-Gomez A, Ocio EM, Crusoe E, Santamaria C, Hernandez-Campo P, Blanco JF et al. Dasatinib as a bone-modifying agent: anabolic and anti-resorptive effects. PLoS One 2012; 7: e34914.
Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N et al. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res 2009; 24: 1194–1205.
Lane NE, Yao W, Nakamua MC, Humphrey MB, Kimmel D, Huang X et al. Mice lacking the integrin beta5 subunit have accelerated osteoclast maturation and increased activity in the estrogen-deficient state. J Bone Miner Res 2005; 20: 58–66.
Hapidin H, Othman F, Soelaiman IN, Shuid AN, Luke DA, Mohamed N . Negative effects of nicotine on bone-resorbing cytokines and bone histomorphometric parameters in male rats. J Bone Miner Metab 2007; 25: 93–98.
Gross S, Piwnica-Worms D . Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods 2005; 2: 607–614.
Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 2004; 22: 2108–2121.
Moreau P, Coiteux V, Hulin C, Leleu X, van de Velde H, Acharya M et al. Prospective comparison of subcutaneous versus intravenous administration of bortezomib in patients with multiple myeloma. Haematologica 2008; 93: 1908–1911.
O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res 2009; 15: 7085–7091.
Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 2004; 104: 2484–2491.
Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 2008; 22: 1925–1932.
Vaananen HK, Laitala-Leinonen T . Osteoclast lineage and function. Arch Biochem Biophys 2008; 473: 132–138.
Nakamura I, Duong le T, Rodan SB, Rodan GA . Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab 2007; 25: 337–344.
Weilbaecher KN, Guise TA, McCauley LK . Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11: 411–425.
Matsumoto T, Abe M . TGF-beta-related mechanisms of bone destruction in multiple myeloma. Bone 2011; 48: 129–134.
Chen G, Deng C, Li Li YP. . TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012; 8: 272–288.
Choi YH, Gu YM, Oh JW, Lee KY . Osterix is regulated by Erk1/2 during osteoblast differentiation. Biochem Biophys Res Commun 2011; 415: 472–478.
Lee KS, Hong SH, Bae SC . Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene 2002; 21: 7156–7163.
Tohmonda T, Miyauchi Y, Ghosh R, Yoda M, Uchikawa S, Takito J et al. The IRE1alpha-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep 2011; 12: 451–457.
Dallas SL, Garrett IR, Oyajobi BO, Dallas MR, Boyce BF, Bauss F et al. Ibandronate reduces osteolytic lesions but not tumor burden in a murine model of myeloma bone disease. Blood 1999; 93: 1697–1706.
Edwards CM, Lwin ST, Fowler JA, Oyajobi BO, Zhuang J, Bates AL et al. Myeloma cells exhibit an increase in proteasome activity and an enhanced response to proteasome inhibition in the bone marrow microenvironment in vivo. Am J Hematol 2009; 84: 268–272.
Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res 2011; 17: 2734–2743.
Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K et al. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene 2002; 21: 1346–1358.
Roccaro AM, Sacco A, Aujay M, Ngo HT, Azab AK, Azab F et al. Selective inhibition of chymotrypsin-like activity of the immunoproteasome and constitutive proteasome in Waldenstrom macroglobulinemia. Blood 2010; 115: 4051–4060.
Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K et al. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun 2005; 333: 200–205.
Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH . Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci USA 2003; 100: 9946–9951.
Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Lee KP, Boise LH . Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006; 107: 4907–4916.
Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 2007; 110: 2641–2649.
Acknowledgements
We are grateful to the Washington University MM/MGUS Research Program and Tissue Bank and to Lindsay Goddard, Montserrat Martín, Isabel Isidro, Teresa Prieto and Almudena Martín for their excellent technical work. This research was supported by grants from the National Institutes of Health (T32CA113275:MAH; P01CA100730:KNW; P50CA94056:DP-W), the St Louis Men’s Group Against Cancer (KNW), the Holway Myeloma Fund (KNW), the Spanish MICINN-ISCIII (PI081825), the Fundación de Investigación Médica Mutua Madrileña (AP27262008), the Centro en Red de Medicina Regenerativa y Terapia Celular de Castilla y León, the Spanish Myeloma Network Program (RD06/0020/0006 and RD06/0020/0041) and Spanish FIS (PS09/01897). MicroCT services were provided by the WU musculoskeletal core (P30AR057235).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CJK is an employee of Onyx Pharmaceuticals. All other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Hurchla, M., Garcia-Gomez, A., Hornick, M. et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia 27, 430–440 (2013). https://doi.org/10.1038/leu.2012.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.183
Keywords
This article is cited by
-
Myeloma bone disease: pathogenesis and management in the era of new anti-myeloma agents
Journal of Bone and Mineral Metabolism (2023)
-
A pilot study of 3D tissue-engineered bone marrow culture as a tool to predict patient response to therapy in multiple myeloma
Scientific Reports (2021)
-
Carfilzomib, dexamethasone, and daratumumab in Asian patients with relapsed or refractory multiple myeloma: post hoc subgroup analysis of the phase 3 CANDOR trial
International Journal of Hematology (2021)
-
ER stress arm XBP1s plays a pivotal role in proteasome inhibition-induced bone formation
Stem Cell Research & Therapy (2020)
-
Carfilzomib with immunomodulatory drugs for the treatment of newly diagnosed multiple myeloma
Leukemia (2019)