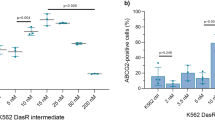
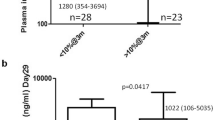
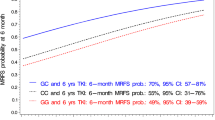
Abstract
Imatinib is a highly effective therapy for chronic phase-chronic myeloid leukaemia (CP-CML) patients; however, responses to frontline imatinib are variable. The human organic cation transporter 1 (OCT1; SLC22A1) has been reported to be the main influx transporter involved in imatinib uptake into CML cells. Furthermore, variation in the efficiency of imatinib influx via OCT1 has been demonstrated to result in the inter-patient variation observed in primary response to imatinib. Although studies have questioned the role of OCT1 in imatinib influx, these have been largely performed in non-clinical settings. Measuring both OCT1 mRNA levels and the functional activity of OCT1 in primary leukaemic cells has been demonstrated to predict molecular response and outcome in imatinib-treated CP-CML patients in several independent studies. Here, the role of OCT1 and OCT1 genetic variants in imatinib uptake and response prediction is summarised and data generated from model systems assessing the role of OCT1 in imatinib transport is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Regenass U et al. Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc Natl Acad Sci USA 1995; 92: 2558–2562.
White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood 2006; 108: 697–704.
White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood 2007; 110: 4064–4072.
Deininger M, O'Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH Annu Meet Abstr 2009; 114: 1126.
Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood 2008; 112: 4437–4444.
Cortes J, Hochhaus A, Hughes T, Kantarjian H . Front-line and salvage therapies with tyrosine kinase inhibitors and other treatments in chronic myeloid leukemia. J Clin Oncol 2011; 29: 524–531.
Kantarjian HM, Talpaz M, O'Brien S, Giles F, Garcia-Manero G, Faderl S et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 2003; 101: 473–475.
Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE . hOCT 1 and resistance to imatinib. Blood 2005; 106: 1133–1134.
Thomas J, Wang L, Clark RE, Pirmohamed M . Active transport of imatinib into and out of cells: implications for drug resistance. Blood 2004; 104: 3739–3745.
White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol 2010; 28: 2761–2767.
Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381–2388.
Zhong JS, Meng FY, Xu D, Zhou HS, Dai M . Correlation between imatinib trough concentration and efficacy in Chinese chronic myelocytic leukemia patients. Acta Haematol 2012; 127: 221–227.
Nardinelli L, Sanabani SS, Didone A, Ferreira Pde B, Serpa M, Novaes MM et al. Pretherapeutic expression of the hOCT1 gene predicts a complete molecular response to imatinib mesylate in chronic-phase chronic myeloid leukemia. Acta Haematol 2012; 127: 228–234.
Gromicho M, Magalhaes M, Torres F, Dinis J, Fernandes AR, Rendeiro P et al. Instability of mRNA expression signatures of drug transporters in chronic myeloid leukemia patients resistant to imatinib. Oncol Rep 2013; 29: 741–750.
Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008; 14: 3141–3148.
Nies AT, Schaeffeler E, van der Kuip H, Cascorbi I, Bruhn O, Kneba M et al. Cellular uptake of imatinib into leukemic cells is independent of human organic cation transporter 1 (OCT1). Clin Cancer Res 2014; 20: 985–994.
Koepsell H, Lips K, Volk C . Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 2007; 24: 1227–1251.
Koepsell H, Endou H . The SLC22 drug transporter family. Pflugers Archiv 2004; 447: 666–676.
White DL, Saunders VA, Dang P, Engler J, Hughes TP . OCT-1 activity measurement provides a superior imatinib response predictor than screening for single-nucleotide polymorphisms of OCT-1. Leukemia 2010; 24: 1962–1965.
White DL, Hughes TP . Classification of patients with chronic myeloid leukemia on basis of BCR-ABL transcript level at 3 months fails to identify patients with low organic cation transporter-1 activity destined to have poor imatinib response. J Clin Oncol 2012; 30: 1144–1145.
Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE . Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther 2008; 83: 258–264.
Giannoudis A, Wang L, Jorgensen AL, Xinarianos G, Davies A, Pushpakom S et al. The hOCT1 SNPs M420del and M408V alter imatinib uptake and M420del modifies clinical outcome in imatinib-treated chronic myeloid leukemia. Blood 2013; 121: 628–637.
Zhang WW, Cortes JE, Yao H, Zhang L, Reddy NG, Jabbour E et al. Predictors of primary imatinib resistance in chronic myelogenous leukemia are distinct from those in secondary imatinib resistance. J Clin Oncol 2009; 27: 3642–3649.
de Lima LT, Vivona D, Bueno CT, Hirata RD, Hirata MH, Luchessi AD et al. Reduced ABCG2 and increased SLC22A1 mRNA expression are associated with imatinib response in chronic myeloid leukemia. Med Oncol 2014; 31: 851.
Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH, Lee JJ et al. OCT-1, ABCB1, and ABCG2 expression in imatinib-resistant chronic myeloid leukemia treated with dasatinib or nilotinib. Chonnam Med J 2014; 50: 102–111.
Burger H, Mathijssen RH, Sparreboom A, Wiemer EA . Can "specific" OCT1 inhibitors be used to determine OCT1 transporter activity toward imatinib? Blood 2013; 121: 4965–4966.
Ahlin G, Karlsson J, Pedersen JM, Gustavsson L, Larsson R, Matsson P et al. Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1. J Med Chem 2008; 51: 5932–5942.
White DL, Radich J, Soverini S, Saunders VA, Frede AK, Dang P et al. Chronic phase chronic myeloid leukemia patients with low OCT-1 activity randomized to high-dose imatinib achieve better responses and have lower failure rates than those randomized to standard-dose imatinib. Haematologica 2012; 97: 907–914.
White DL, Saunders V, Frede A, GrootObbink K, Slader C, Yeung DT et al. Early switching from imatinib to nilotinib in CML patients failing to achieve early molecular targets may not be an effective approach in patients with very low OCT-1 activity: A TIDEL II Sub-Study. ASH Annu Meet Abstr 2010; 116: 356.
White DL, Saunders VA, Yeung DT, Grigg A, Hughes TP . Early molecular response to imatinib in CP-CML patients: the significance of early dose intensity and OCT-1 activity in responders and efficacy of dose escalation and switch to nilotinib in non-responders. ASH Annu Meet Abstr 2012; 120: 693.
Davies A, Jordanides NE, Giannoudis A, Lucas CM, Hatziieremia S, Harris RJ et al. Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia 2009; 23: 1999–2006.
Engler JR, Frede A, Saunders VA, Zannettino AC, Hughes TP, White DL . Chronic myeloid leukemia CD34+ cells have reduced uptake of imatinib due to low OCT-1 activity. Leukemia 2010; 24: 765–770.
Ciarimboli G, Schlatter E . Regulation of organic cation transport. Pflugers Archiv 2005; 449: 423–441.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419.
Greenbaum D, Colangelo C, Williams K, Gerstein M . Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 2003; 4: 117.
Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, Uhlen M et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 2009; 10: 365.
Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ . Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 2011; 121: 396–409.
Engler JR, Frede A, Saunders V, Zannettino A, White DL, Hughes TP . The poor response to imatinib observed in CML patients with low OCT-1 activity is not attributable to lower uptake of imatinib into their CD34+ cells. Blood 2010; 116: 2776–2778.
Engler JR, Zannettino AC, Bailey CG, Rasko JE, Hughes TP, White DL . OCT-1 function varies with cell lineage but is not influenced by BCR-ABL. Haematologica 2011; 96: 213–220.
Watkins DB, Kok CH, Hughes TP, Slader C, D'Andrea RJ, White DL . Differential lineage involvement between very low and higher OCT-1 activity chronic-phase CML patients. ASH Annu Meet Abstr 2011; 118, Abstract 1675.
Nie W, Sweetser S, Rinella M, Green RM . Transcriptional regulation of murine Slc22a1 (Oct1) by peroxisome proliferator agonist receptor-alpha and -gamma. Am J Physiol Gastrointest Liver Physiol 2005; 288: G207–G212.
Wang L, Giannoudis A, Austin G, Clark RE . Peroxisome proliferator-activated receptor activation increases imatinib uptake and killing of chronic myeloid leukemia cells. Exp Hematol. 2012; 40: 811–819 e2.
Wang J, Kok C, D'Andrea R, Hughes T, White D . Role Of peroxisome proliferator-activated receptor gamma (PPARγ) and its ligands in the regulation of functional OCT-1 activity in CML cells. ASH Annu Meet Abstr 2013; 21, Abstract 1470.
Kerb R, Brinkmann U, Chatskaia N, Gorbunov D, Gorboulev V, Mornhinweg E et al. Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics 2002; 12: 591–595.
Shu Y, Leabman MK, Feng B, Mangravite LM, Huang CC, Stryke D et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci USA 2003; 100: 5902–5907.
Itoda M, Saito Y, Maekawa K, Hichiya H, Komamura K, Kamakura S et al. Seven novel single nucleotide polymorphisms in the human SLC22A1 gene encoding organic cation transporter 1 (OCT1). Drug Metab Pharmacokinet 2004; 19: 308–312.
Sakata T, Anzai N, Shin HJ, Noshiro R, Hirata T, Yokoyama H et al. Novel single nucleotide polymorphisms of organic cation transporter 1 (SLC22A1) affecting transport functions. Biochem Biophys Res Commun 2004; 313: 789–793.
Leabman MK, Huang CC, DeYoung J, Carlson EJ, Taylor TR, de la Cruz M et al. Natural variation in human membrane transporter genes reveals evolutionary and functional constraints. Proc Natl Acad Sci USA 2003; 100: 5896–5901.
Matakidou A, Eisen T, Houlston RS . TP53 polymorphisms and lung cancer risk: a systematic review and meta-analysis. Mutagenesis 2003; 18: 377–385.
Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S . A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics 2008; 9: 361–374.
Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF . A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1999; 8: 843–854.
Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 2008; 83: 273–280.
Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007; 117: 1422–1431.
Takane H, Shikata E, Otsubo K, Higuchi S, Ieiri I . Polymorphism in human organic cation transporters and metformin action. Pharmacogenomics 2008; 9: 415–422.
Angelini S, Soverini S, Ravegnini G, Barnett M, Turrini E, Thornquist M et al. Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy. Haematologica 2013; 98: 193–200.
Bazeos A, Marin D, Reid AG, Gerrard G, Milojkovic D, May PC et al. hOCT1 transcript levels and single nucleotide polymorphisms as predictive factors for response to imatinib in chronic myeloid leukemia. Leukemia 2010; 24: 1243–1245.
Di Paolo A, Polillo M, Capecchi M, Cervetti G, Barate C, Angelini S et al. The c.480C>G polymorphism of hOCT1 influences imatinib clearance in patients affected by chronic myeloid leukemia. Pharmacogenomics J 2014; 14: 328–335.
Grinfeld J, Gerrard G, Alikian M, Alonso-Dominguez J, Ale S, Valganon M et al. A common novel splice variant of SLC22A1 (OCT1) is associated with impaired responses to imatinib in patients with chronic myeloid leukaemia. Br J Haematol 2013; 163: 631–639.
Kim DH, Sriharsha L, Xu W, Kamel-Reid S, Liu X, Siminovitch K et al. Clinical relevance of a pharmacogenetic approach using multiple candidate genes to predict response and resistance to imatinib therapy in chronic myeloid leukemia. Clin Cancer Res 2009; 15: 4750–4758.
Koren-Michowitz M, Buzaglo Z, Ribakovsky E, Schwarz M, Pessach I, Shimoni A et al. OCT1 genetic variants are associated with long term outcomes in imatinib treated chronic myeloid leukemia patients. Eur J Haematol 2014; 92: 283–288.
Maffioli M, Camos M, Gaya A, Hernandez-Boluda JC, Alvarez-Larran A, Domingo A et al. Correlation between genetic polymorphisms of the hOCT1 and MDR1 genes and the response to imatinib in patients newly diagnosed with chronic-phase chronic myeloid leukemia. Leuk Res 2011; 35: 1014–1019.
Takahashi N, Miura M, Scott SA, Kagaya H, Kameoka Y, Tagawa H et al. Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J Hum Genetics 2010; 55: 731–737.
Seong SJ, Lim M, Sohn SK, Moon JH, Oh SJ, Kim BS et al. Influence of enzyme and transporter polymorphisms on trough imatinib concentration and clinical response in chronic myeloid leukemia patients. Ann Oncol 2013; 24: 756–760.
Singh O, Chan JY, Lin K, Heng CC, Chowbay B . SLC22A1-ABCB1 haplotype profiles predict imatinib pharmacokinetics in Asian patients with chronic myeloid leukemia. PLoS ONE 2012; 7: e51771.
Vine J, Cohen SB, Ruchlemer R, Goldschmidt N, Levin M, Libster D et al. Polymorphisms in the human organic cation transporter and the multidrug resistance gene: correlation with imatinib levels and clinical course in patients with chronic myeloid leukemia. Leuk Lymphoma 2014; 55: 2525–2531.
Zach O, Krieger O, Foedermayr M, Zellhofer B, Lutz D . OCT1 (SLC22A1) R61C polymorphism and response to imatinib treatment in chronic myeloid leukemia patients. Leuk Lymphoma 2008; 49: 2222–2223.
Tzvetkov MV, Seitz T, Bokelmann K, Mueller T, Brockmoller J, Koepsell H . Does the haplotype Met408-Del420, which was apparently predictive for imatinib efficacy, really exist and how strongly may it affect OCT1 activity? Blood 2014; 123: 1427–1429.
Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 2004; 22: 935–942.
Nambu T, Hamada A, Nakashima R, Yuki M, Kawaguchi T, Mitsuya H et al. Association of SLCO1B3 polymorphism with intracellular accumulation of imatinib in leukocytes in patients with chronic myeloid leukemia. Biol Pharm Bull 2011; 34: 114–119.
Eadie L, Hughes TP, White DL . Nilotinib does not significantly reduce imatinib OCT-1 activity in either cell lines or primary CML cells. Leukemia 2010; 24: 855–857.
Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res 2008; 14: 3881–3888.
Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genetics 2004; 36: 471–475.
Ohashi R, Tamai I, Yabuuchi H, Nezu JI, Oku A, Sai Y et al. Na(+)-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther 1999; 291: 778–784.
Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M et al. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther 1999; 289: 768–773.
Zhang L, Schaner ME, Giacomini KM . Functional characterization of an organic cation transporter (hOCT1) in a transiently transfected human cell line (HeLa). J Pharmacol Exp Ther 1998; 286: 354–361.
Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ et al. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am J Physiol Renal Physiol 2000; 279: F449–F458.
Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T et al. Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochem Biophys Res Commun 2005; 333: 754–762.
Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol 1998; 54: 342–352.
Suhre WM, Ekins S, Chang C, Swaan PW, Wright SH . Molecular determinants of substrate/inhibitor binding to the human and rabbit renal organic cation transporters hOCT2 and rbOCT2. Mol Pharmacol 2005; 67: 1067–1077.
Amphoux A, Vialou V, Drescher E, Bruss M, Mannoury La Cour C, Rochat C et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 2006; 50: 941–952.
Hayer-Zillgen M, Bruss M, Bonisch H . Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol 2002; 136: 829–836.
Grundemann D, Koster S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F et al. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J Biol Chem 1998; 273: 30915–30920.
Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 1997; 16: 871–881.
Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM . Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol 1997; 51: 913–921.
Acknowledgements
We thank Dr Jackie Wang for helpful discussion and manuscript reviewing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TPH received Honoraria, Membership on an entity's Board of Directors or advisory committees and Research Funding from Novartis and BMS; Honoraria, Membership on an entity's Board of Directors or advisory committees from Ariad; Membership on an entity's Board of Directors or advisory committees from Pfizer. DLW received Honoraria and Research Funding from Novartis and BMS, Research Funding from Ariad. DBW has no conflict of interest to disclose. All authors together wrote the review article.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Watkins, D., Hughes, T. & White, D. OCT1 and imatinib transport in CML: is it clinically relevant?. Leukemia 29, 1960–1969 (2015). https://doi.org/10.1038/leu.2015.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.170
This article is cited by
-
Systematic review and meta-analysis of standard-dose imatinib vs. high-dose imatinib and second generation tyrosine kinase inhibitors for chronic myeloid leukemia
Journal of Cancer Research and Clinical Oncology (2017)
-
Association of the hOCT1/ABCB1 genotype with efficacy and tolerability of imatinib in patients affected by chronic myeloid leukemia
Cancer Chemotherapy and Pharmacology (2017)
-
Pharmacogenetics and Pharmacogenomics of Targeted Therapeutics in Chronic Myeloid Leukemia
Molecular Diagnosis & Therapy (2017)
-
hOCT1 gene expression predict for optimal response to Imatinib in Tunisian patients with chronic myeloid leukemia
Cancer Chemotherapy and Pharmacology (2017)