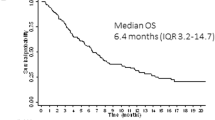
Abstract
Decitabine may open the chromatin structure of leukemia cells making them accessible to the calicheamicin epitope of gemtuzumab ozogamicin (GO). A total of 110 patients (median age 70 years; range 27–89 years) were treated with decitabine and GO in a trial designed on model-based futility to accommodate subject heterogeneity: group 1: relapsed/refractory acute myeloid leukemia (AML) with complete remission duration (CRD) <1 year (N=28, 25%); group 2: relapsed/refractory AML with CRD ⩾1 year (N=5, 5%); group 3: untreated AML unfit for intensive chemotherapy or untreated myelodysplastic syndrome (MDS) or untreated myelofibrosis (MF; N=57, 52%); and group 4: AML evolving from MDS or relapsed/refractory MDS or MF (N=20, 18%). Treatment consisted of decitabine 20 mg/m2 daily for 5 days and GO 3 mg/m2 on day 5. Post-induction therapy included five cycles of decitabine+GO followed by decitabine alone. Complete remission (CR)/CR with incomplete count recovery was achieved in 39 (35%) patients; group 1= 5/28 (17%), group 2=3/5 (60%), group 3=24/57 (42%) and group 4=7/20 (35%). The 8-week mortality in groups 3 and 4 was 16% and 10%, respectively. Common drug-related adverse events included nausea, mucositis and hemorrhage. Decitabine and GO improved the response rate but not overall survival compared with historical outcomes in untreated AML ⩾60 years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kantarjian HM . Therapy for elderly patients with acute myeloid leukemia: a problem in search of solutions. Cancer 2007; 109: 1007–1010.
Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006; 106: 1090–1098.
Nazha A, Ravandi F . Acute myeloid leukemia in the elderly: do we know who should be treated and how? Leuk Lymphoma 2014; 55: 979–987.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE et al. Age and acute myeloid leukemia. Blood 2006; 107: 3481–3485.
Tallman MS, Gilliland DG, Rowe JM . Drug therapy for acute myeloid leukemia. Blood 2005; 106: 1154–1163.
Estey EH . Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia 2000; 14: 476–479.
Ravandi F, Cortes J, Faderl S, O’Brien S, Garcia-Manero G, Verstovsek S et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010; 116: 5818–5823, quiz 6153.
Stanisic S, Kalaycio M . Treatment of refractory and relapsed acute myelogenous leukemia. Expert Rev Anticancer Ther 2002; 2: 287–295.
Scheinberg DA, Lovett D, Divgi CR, Graham MC, Berman E, Pentlow K et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol 1991; 9: 478–490.
Appelbaum FR, Matthews DC, Eary JF, Badger CC, Kellogg M, Press OW et al. The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation 1992; 54: 829–833.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001; 19: 3244–3254.
Zein N, Poncin M, Nilakantan R, Ellestad GA . Calicheamicin gamma 1I and DNA: molecular recognition process responsible for site-specificity. Science 1989; 244: 697–699.
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 2011; 29: 369–377.
Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012; 379: 1508–1516.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012; 30: 3924–3931.
Delaunay J, Recher C, Pigneux A, Witz F, Vey N, Blanchet O et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR Study. Blood 2011; 118: 37–38.
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001; 7: 1490–1496.
Christman JK . 5-Azacytidine and 5-aza-2'-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 2002; 21: 5483–5495.
Karpf AR, Jones DA . Reactivating the expression of methylation silenced genes in human cancer. Oncogene 2002; 21: 5496–5503.
Jones PA, Taylor SM . Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980; 20: 85–93.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30: 2670–2677.
Balaian L, Ball ED . Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia 2006; 20: 2093–2101.
Goodman PA, Burkhardt N, Juran B, Tibbles HE, Uckun FM . Hypermethylation of the spleen tyrosine kinase promoter in T-lineage acute lymphoblastic leukemia. Oncogene 2003; 22: 2504–2514.
Yuan Y, Mendez R, Sahin A, Dai JL . Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res 2001; 61: 5558–5561.
Kurimoto M, Matsuoka H, Hanaoka N, Uneda S, Murayama T, Sonoki T et al. Pretreatment of leukemic cells with low-dose decitabine markedly enhances the cytotoxicity of gemtuzumab ozogamicin. Leukemia 2013; 27: 233–235.
Nand S, Othus M, Godwin JE, Willman CL, Norwood TH, Howard DS et al. A phase 2 trial of azacitidine and gemtuzumab ozogamicin therapy in older patients with acute myeloid leukemia. Blood 2013; 122: 3432–3439.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010; 115: 1703–1708.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001; 19: 3244–3254.
Wathen JK, Thall PF, Cook JD, Estey EH . Accounting for patient heterogeneity in phase II clinical trials. Stati Med 2008; 27: 2802–2815.
Kaplan EL, Meier P . Nonparametric-estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013; 121: 4854–4860.
Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia 2002; 16: 1627–1636.
Larson RA, Sievers EL, Stadtmauer EA, Lowenberg B, Estey EH, Dombret H et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005; 104: 1442–1452.
Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley K et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013; 27: 75–81.
Nand S, Godwin J, Smith S, Barton K, Michaelis L, Alkan S et al. Hydroxyurea, azacitidine and gemtuzumab ozogamicin therapy in patients with previously untreated non-M3 acute myeloid leukemia and high-risk myelodysplastic syndromes in the elderly: results from a pilot trial. Leuk Lymphoma 2008; 49: 2141–2147.
Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML . Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood 2003; 102: 1466–1473.
Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007; 109: 4168–4170.
Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007; 109: 52–57.
Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106: 1794–1803.
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20: 2429–2440.
Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M . A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood 1996; 88: 756.
Malfuson JV, Konopacki J, Thepenier C, Eddou H, Foissaud V, de Revel T . Fractionated doses of gemtuzumab ozogamicin combined with 3+7 induction chemotherapy as salvage treatment for young patients with acute myeloid leukemia in first relapse. Ann Hematol 2012; 91: 1871–1877.
Acknowledgements
We thank Eisai Corporation for supporting the clinical trial. This study was conducted following the guidelines of The University of Texas MD Anderson Cancer Center after local IRB approval. It was supported in part by the MD Anderson Cancer Center Support Grant (CCSG) CA016672 and Eisai Corporation.
Author contributions
ND and GB wrote the paper; GB and HK designed and coordinated the study; MK, SO, AF, SV, TK, EJ, NP, CD, JC, GB, HK enrolled the patients and conducted the research; and ND, GB, XW, SP analyzed the data and performed the statistics. All of the authors participated in the discussion, have reviewed and approved the current version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
GB received research funding from Eisai Corporation. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Daver, N., Kantarjian, H., Ravandi, F. et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia 30, 268–273 (2016). https://doi.org/10.1038/leu.2015.244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.244
This article is cited by
-
Vaccines: a promising therapy for myelodysplastic syndrome
Journal of Hematology & Oncology (2024)
-
Double and single mixed-lineage leukemia-rearranged subclones in pediatric acute myeloid leukemia: a case report
Journal of Medical Case Reports (2021)
-
Somatostatin receptor mediated targeting of acute myeloid leukemia by photodynamic metal complexes for light induced apoptosis
Scientific Reports (2020)
-
Gemtuzumab ozogamicin and novel antibody-drug conjugates in clinical trials for acute myeloid leukemia
Biomarker Research (2019)
-
HO-1 promotes resistance to an EZH2 inhibitor through the pRB-E2F pathway: correlation with the progression of myelodysplastic syndrome into acute myeloid leukemia
Journal of Translational Medicine (2019)