Abstract
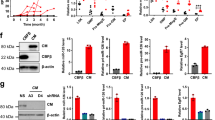
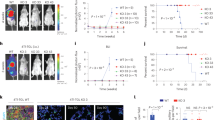
The PD-L1/PD-1 pathway is a critical component of the immunosuppressive tumor microenvironment in acute myeloid leukemia (AML), but little is known about its regulation. We investigated the role of the MUC1 oncoprotein in modulating PD-L1 expression in AML. Silencing of MUC1 in AML cell lines suppressed PD-L1 expression without a decrease in PD-L1 mRNA levels, suggesting a post-transcriptional mechanism of regulation. We identified the microRNAs miR-200c and miR-34a as key regulators of PD-L1 expression in AML. Silencing of MUC1 in AML cells led to a marked increase in miR-200c and miR-34a levels, without changes in precursor microRNA, suggesting that MUC1 might regulate microRNA-processing. MUC1 signaling decreased the expression of the microRNA-processing protein DICER, via the suppression of c-Jun activity. NanoString (Seattle, WA, USA) array of MUC1-silenced AML cells demonstrated an increase in the majority of probed microRNAs. In an immunocompetent murine AML model, targeting of MUC1 led to a significant increase in leukemia-specific T cells. In concert, targeting MUC1 signaling in human AML cells resulted in enhanced sensitivity to T-cell-mediated lysis. These findings suggest MUC1 is a critical regulator of PD-L1 expression via its effects on microRNA levels and represents a potential therapeutic target to enhance anti-tumor immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Teague RM, Kline J . Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer 2013; 1;, doi:10.1186/2051-1426-1-13
Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood 2009; 114: 3909–3916.
Coles SJ, Gilmour MN, Reid R, Knapper S, Burnett AK, Man S et al. The immunosuppressive ligands PD-L1 and CD200 are linked in AML T-cell immunosuppression: identification of a new immunotherapeutic synapse. Leukemia 2015; 29: 1952–1954.
Wang X, Li J, Dong K, Lin F, Long M, Ouyang Y et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal 2015; 27: 443–452.
Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N et al. In acute myeloid leukemia, B7-H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunol Immunother 2010; 59: 1839–1849.
Zhang L, Gajewski TF, Kline J . PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009; 114: 1545–1552.
McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase ia study. J Clin Oncol 2016; 34: 833–842.
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311–319.
Takahashi T, Makiguchi Y, Hinoda Y, Kakiuchi H, Nakagawa N, Imai K et al. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol 1994; 153: 2102–2109.
Burton J, Mishina D, Cardillo T, Lew K, Rubin A, Goldenberg DM et al. Epithelial mucin-1 (MUC1) expression and MA5 anti-MUC1 monoclonal antibody targeting in multiple myeloma. Clin Cancer Res 1999; 5: 3065s–3072s.
Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A et al. Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood 1999; 93: 1287–1298.
Paydaş S, Sahin B, Gönlüşen G, Hazar B, Zorludemir S . MUC1 expression in plasmacytoma. Leuk Res 2001; 25: 221–225.
Cloosen S, Gratama J, van Leeuwen EBM, Senden-Gijsbers BLMG, Oving EBH, von Mensdorff-Pouilly S et al. Cancer specific Mucin-1 glycoforms are expressed on multiple myeloma. Br J Haematol 2006; 135: 513–516.
Baldus SE, Palmen C, Thiele J . MUC1 (EMA) expressing plasma cells in bone marrow infiltrated by plasma cell myeloma. Histol Histopathol 2007; 22: 889–893.
Tan Z, Huang Q, Zang J, Teng S-F, Chen T-R, Wei H et al. HIF-1α activates hypoxia-induced BCL-9 expression in human colorectal cancer cells. Oncotarget 2017; 8: 25885–25896.
Bouillez A, Rajabi H, Pitroda S, Jin C, Alam M, Kharbanda A et al. Inhibition of MUC1-C suppresses MYC expression and attenuates malignant growth in KRAS mutant lung adenocarcinomas. Cancer Res 2016; 76: 1538–1548.
Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S et al. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol 2007; 9: 1419–1427.
Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S et al. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res 2009; 69: 7013–7021.
Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R et al. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene 2015; 34: 5187–5197.
Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C et al. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene 2010; 29: 920–929.
Ahmad R, Rajabi H, Kosugi M, Joshi MD, Alam M, Vasir B et al. MUC1-C oncoprotein promotes STAT3 activation in an autoinductive regulatory loop. Sci Signal 2011; 4: ra9.
Yin L, Ahmad R, Kosugi M, Kufe T, Vasir B, Avigan D et al. Survival of human multiple myeloma cells is dependent on MUC1 C-terminal transmembrane subunit oncoprotein function. Mol Pharmacol 2010; 78: 166–174.
Stroopinsky D, Rosenblatt J, Ito K, Mills H, Yin L, Rajabi H et al. MUC1 is a potential target for the treatment of acute myeloid leukemia stem cells. Cancer Res 2013; 73: 5569–5579.
Yin L, Wu Z, Avigan D, Rosenblatt J, Stone R, Kharbanda S et al. MUC1-C oncoprotein suppresses reactive oxygen species-induced terminal differentiation of acute myelogenous leukemia cells. Blood 2011; 117: 4863–4870.
Yin L, Kosugi M, Kufe D . Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by down-regulating TIGAR expression and depleting NADPH. Blood 2012; 119: 810–816.
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP . The impact of microRNAs on protein output. Nature 2008; 455: 64–71.
Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5: 5241.
Rosenblatt J, Wu Z, Vasir B, Zarwan C, Stone R, Mills H et al. Generation of tumor-specific T lymphocytes using dendritic cell/tumor fusions and anti-CD3/CD28. J Immunother 2010; 33: 155–166.
Panchamoorthy G, Rehan H, Kharbanda A, Ahmad R, Kufe D . A monoclonal antibody against the oncogenic mucin 1 cytoplasmic domain. Hybrid 2011; 30: 531–535.
Kang S, Tsai LT, Zhou Y, Evertts A, Xu S, Griffin MJ et al. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nat Cell Biol 2015; 17: 44–56.
UCSC. UCSC Genome Browser. Available at http://genome.ucsc.edu/.
Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P . c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 1988; 55: 917–924.
Kimchi ET, Posner MC, Park JO, Darga TE, Kocherginsky M, Karrison T et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res 2005; 65: 3146–3154.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300.
Gazon H, Belrose G, Terol M, Meniane J-C, Mesnard J-M, Césaire R et al. Impaired expression of DICER and some microRNAs in HBZ expressing cells from acute adult T-cell leukemia patients. Oncotarget 2016; 7: 30258–30275.
Selever J, Gu G, Lewis MT, Beyer A, Herynk MH, Covington KR et al. Dicer-mediated upregulation of BCRP confers tamoxifen resistance in human breast cancer cells. Clin Cancer Res 2011; 17: 6510–6521.
Leppä S, Saffrich R, Ansorge W, Bohmann D . Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J 1998; 17: 4404–4413.
Deng Z, Sui G, Rosa PM, Zhao W . Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling pathway in microglial cells. PLoS One 2012; 7: e36739.
Zhang W, Han D, Wan P, Pan P, Cao Y, Liu Y et al. ERK/c-Jun recruits Tet1 to induce Zta expression and Epstein-Barr virus reactivation through DNA demethylation. Sci Rep 2016; 6: 34543.
Herdegen T, Claret FX, Kallunki T, Martin-Villalba A, Winter C, Hunter T et al. Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J Neurosci 1998; 18: 5124–5135.
Yarza R, Vela S, Solas M, Ramirez MJ . c-Jun N-terminal kinase (JNK) Signaling as a therapeutic target for Alzheimer’s disease. Front Pharmacol 2016; 6: 321.
Rosenblatt J, Stone R, Uhl L, Neuberg D, Vasir B, Somaiya P et al Clinical trial evaluating DC/AML fusion cell vaccination in AML patients who achieve a chemotherapy-induced remission, Vol 122. ASH Annual Meeting; New Orleans Convention Center: New Orleans, LI, USA, 2013 American Society of Hematology. p 3928.
Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother 2011; 34: 409–418.
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211: 781–790.
Christiansson L, Söderlund S, Svensson E, Mustjoki S, Bengtsson M, Simonsson B et al. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in sokal high risk chronic myeloid leukemia. PLoS One 2013; 8: e55818.
Napolitano M, D’Alterio C, Cardone E, Trotta AM, Pecori B, Rega D et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 2015; 6: 8261–8270.
Kufe DW . Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther 2009; 8: 1197–1203.
Tagde A, Rajabi H, Bouillez A, Alam M, Gali R, Bailey S et al. MUC1-C drives MYC in multiple myeloma. Blood 2016; 127: 2587–2597.
Liu S, Yin L, Stroopinsky D, Rajabi H, Puissant A, Stegmaier K et al. MUC1-C oncoprotein promotes FLT3 receptor activation in acute myeloid leukemia cells. Blood 2014; 123: 734–742.
David JM, Hamilton DH, Palena C . MUC1 upregulation promotes immune resistance in tumor cells undergoing brachyury-mediated epithelial-mesenchymal transition. Oncoimmunology 2016; 5: e1117738.
Bar-Natan M, Stroopinsky D, Luptakova K, Coll MD, Apel A, Rajabi H et al. Bone marrow stroma protects myeloma cells from cytotoxic damage via induction of the oncoprotein MUC1. Br J Haematol 2017; 176: 929–938.
Yu Z, Li Y, Fan H, Liu Z, Pestell RG . miRNAs regulate stem cell self-renewal and differentiation. Front Genet 2012; 3: 191.
Kim S, Song JH, Kim S, Qu P, Martin BK, Sehareen WS et al. Loss of oncogenic miR-155 in tumor cells promotes tumor growth by enhancing C/EBP-β-mediated MDSC infiltration. Oncotarget 2016; 7: 11094–11112.
Krill KT, Gurdziel K, Heaton JH, Simon DP, Hammer GD . Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex. Mol Endocrinol 2013; 27: 754–768.
Connerty P, Ahadi A, Hutvagner G . RNA binding proteins in the miRNA pathway. Int J Mol Sci 2015; 17: E31.
Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005; 436: 740–744.
Alam M, Ahmad R, Rajabi H, Kharbanda A, Kufe D . MUC1-C oncoprotein activates ERK→C/EBPβ signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. J Biol Chem 2013; 288: 30892–30903.
Wang J, Liu G, Li Q, Wang F, Xie F, Zhai R et al. Mucin1 promotes the migration and invasion of hepatocellular carcinoma cells via JNK-mediated phosphorylation of Smad2 at the C-terminal and linker regions. Oncotarget 2015; 6: 19264–19278.
Li Q, Liu G, Yuan H, Wang J, Guo Y, Chen T et al. Mucin1 shifts Smad3 signaling from the tumor-suppressive pSmad3C/p21<sup>WAF1</sup> pathway to the oncogenic pSmad3L/c-Myc pathway by activating JNK in human hepatocellular carcinoma cells. Oncotarget 2015; 6: 4253–4265.
Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 2005; 19: 489–501.
Khoshnaw SM, Rakha EA, Abdel-Fatah TM, Nolan CC, Hodi Z, Macmillan DR et al. Loss of Dicer expression is associated with breast cancer progression and recurrence. Breast Cancer Res Treat 2012; 135: 403–413.
Swahari V, Nakamura A, Deshmukh M . The paradox of dicer in cancer. Mol Cell Oncol 2016; 3: e1155006.
Esquela-Kerscher A, Slack FJ . Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269.
Pyzer AR, Stroopinsky D, Rajabi H, Washington A, Tagde A, Coll M et al. MUC1 mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia. Blood 2017; 129: 1791–1801.
Freeman-Keller M, Weber JS . Anti-programmed death receptor 1 immunotherapy in melanoma: rationale, evidence and clinical potential. Ther Adv Med Oncol 2015; 7: 12–21.
Li XJ, Ren ZJ, Tang JH . MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis 2014; 5: e1327.
Acknowledgements
Research was supported by NIDDK P30DK046200. AJ, EA and FJS were supported by a grant from the Ludwig Center at Harvard. This study was funded, in part, by a Chief Academic Officer (CAO) Pilot Grant (2015) awarded to FS and DA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DK holds equity in Genus Oncology and is a consultant to the company. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Pyzer, A., Stroopinsky, D., Rosenblatt, J. et al. MUC1 inhibition leads to decrease in PD-L1 levels via upregulation of miRNAs. Leukemia 31, 2780–2790 (2017). https://doi.org/10.1038/leu.2017.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.163
This article is cited by
-
Targeting PD-1/PD-L1 pathway in myelodysplastic syndromes and acute myeloid leukemia
Experimental Hematology & Oncology (2022)
-
The multifaceted role of MUC1 in tumor therapy resistance
Clinical and Experimental Medicine (2022)
-
Identification of STXBP6-IRF1 positive feedback loop in regulation of PD-L1 in cancer
Cancer Immunology, Immunotherapy (2021)
-
The comprehensive landscape of miR-34a in cancer research
Cancer and Metastasis Reviews (2021)
-
The role of cancer-derived microRNAs in cancer immune escape
Journal of Hematology & Oncology (2020)