Abstract
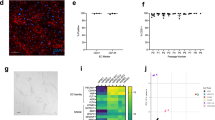
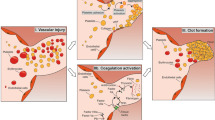
The BCR/ABL1 inhibitor Nilotinib is increasingly used to treat patients with chronic myeloid leukemia (CML). Although otherwise well-tolerated, Nilotinib has been associated with the occurrence of progressive arterial occlusive disease (AOD). Our objective was to determine the exact frequency of AOD and examine in vitro and in vivo effects of Nilotinib and Imatinib on endothelial cells to explain AOD-development. In contrast to Imatinib, Nilotinib was found to upregulate pro-atherogenic adhesion-proteins (ICAM-1, E-selectin, VCAM-1) on human endothelial cells. Nilotinib also suppressed endothelial cell proliferation, migration and tube-formation and bound to a distinct set of target-kinases, relevant to angiogenesis and atherosclerosis, including angiopoietin receptor-1 TEK, ABL-2, JAK1 and MAP-kinases. Nilotinib and siRNA against ABL-2 also suppressed KDR expression. In addition, Nilotinib augmented atherosclerosis in ApoE−/− mice and blocked reperfusion and angiogenesis in a hindlimb-ischemia model of arterial occlusion, whereas Imatinib showed no comparable effects. Clinically overt AOD-events were found to accumulate over time in Nilotinib-treated patients. After a median observation-time of 2.0 years, the AOD-frequency was higher in these patients (29.4%) compared to risk factor- and age-matched controls (<5%). Together, Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on vascular endothelial cells, which may contribute to development of AOD in patients with CML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM . The biology of chronic myeloid leukemia. N Engl J Med 1999; 341: 164–172.
Rowley JD . Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973; 243: 290–293.
Sattler M, Griffin JD . Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol 2003; 40: 4–10.
Druker BJ . Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene 2002; 21: 8541–8546.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004.
Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355: 2408–2417.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–880.
Copland M, Jorgensen HG, Holyoake TL . Evolving molecular therapy for chronic myeloid leukaemia–are we on target? Hematology 2005; 10: 349–359.
Deininger MW . Optimizing therapy of chronic myeloid leukemia. Exp Hematol 2007; 35: 144–154.
Martinelli G, Soverini S, Rosti G, Cilloni D, Baccarani M . New tyrosine kinase inhibitors in chronic myeloid leukemia. Haematologica 2005; 90: 534–541.
Cortes J, Kantarjian H . Beyond dose escalation: clinical options for relapse or resistance in chronic myelogenous leukemia. J Natl Compr Canc Netw 2008; 6: S22–S30.
O'Hare T, Walters DK, Deininger MW, Druker BJ . AMN107: tightening the grip of imatinib. Cancer Cell 2005; 7: 117–119.
Saglio G, Ulisciani S, Bosa M, Cilloni D, Rege-Cambrin G . New therapeutic approaches and prognostic factors in chronic myeloid leukemia. Leuk Lymphoma 2008; 49: 625–628.
Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005; 7: 129–141.
Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006; 354: 2542–2551.
Rosti G, Castagnetti F, Gugliotta G, Palandri F, Martinelli G, Baccarani M . Dasatinib and nilotinib in imatinib-resistant Philadelphia-positive chronic myelogenous leukemia: a 'head-to-head comparison'. Leuk Lymphoma 2010; 51: 583–591.
Rosti G, Palandri F, Castagnetti F, Breccia M, Levato L, Gugliotta G et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood 2009; 114: 4933–4938.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Quintas-Cardama A, Cortes J . Nilotinib: a phenylamino-pyrimidine derivative with activity against BCR-ABL, KIT and PDGFR kinases. Future Oncol 2008; 4: 611–621.
Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007; 110: 4055–4063.
Stover EH, Chen J, Lee BH, Cools J, McDowell E, Adelsperger J et al. The small molecule tyrosine kinase inhibitor AMN107 inhibits TEL-PDGFRbeta and FIP1L1-PDGFRalpha in vitro and in vivo. Blood 2005; 106: 3206–3213.
Racil Z, Razga F, Drapalova J, Buresova L, Zackova D, Palackova M et al. Mechanism of impaired glucose metabolism during nilotinib therapy in patients with chronic myelogenous leukemia. Haematologica 2013; 98: e124–e126.
Rea D, Mirault T, Cluzeau T, Gautier JF, Guilhot F, Dombret H et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica 2014; 99: 1197–1203.
Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol 2011; 86: 533–539.
Giles FJ, Mauro MJ, Hong F, Ortmann CE, McNeill C, Woodman RC et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia 2013; 27: 1310–1315.
Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 2013; 27: 1316–1321.
Le Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst 2011; 103: 1347–1348.
Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S . Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol 2013; 90: 531–532.
Quintas-Cardama A, Kantarjian H, Cortes J . Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk 2012; 12: 337–340.
Tefferi A, Letendre L . Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Hematol 2011; 86: 610–611.
Alhawiti N, Burbury KL, Kwa FA, O'Malley CJ, Shuttleworth P, Alzard M et al. The tyrosine kinase inhibitor, nilotinib potentiates a prothrombotic state. Thromb Res 2016; 145: 54–64.
El-Agamy DS . Nilotinib attenuates endothelial dysfunction and liver damage in high-cholesterol-fed rabbits. Hum Exp Toxicol 2016; pii:0960327116681649.
Katgi A, Sevindik OG, Gokbulut AA, Ozsan GH, Yuksel F, Solmaz SM et al. Nilotinib does not alter the secretory functions of carotid artery endothelial cells in a prothrombotic or antithrombotic fashion. Clin Appl Thromb Hemost 2015; 21: 678–683.
Mian A, Rafiei A, Metodieva A, Haberbosch I, Zeifman A, Titov I et al. PF-114, a novel selective Pan BCR/ABL inhibitor targets the T315I and suppress models of advanced Ph+ ALL. Blood 2013; 122: 3907.
Blankenberg S, Barbaux S, Tiret L . Adhesion molecules and atherosclerosis. Atherosclerosis 2003; 170: 191–203.
Hulthe J, Wikstrand J, Mattsson-Hulten L, Fagerberg B . Circulating ICAM-1 (intercellular cell-adhesion molecule 1) is associated with early stages of atherosclerosis development and with inflammatory cytokines in healthy 58-year-old men: the atherosclerosis and insulin resistance (AIR) study. Clin Sci (Lond) 2002; 103: 123–129.
Bankl HC, Grossschmidt K, Pikula B, Bankl H, Lechner K, Valent P . Mast cells are augmented in deep vein thrombosis and express a profibrinolytic phenotype. Hum Pathol 1999; 30: 188–194.
Sillaber C, Baghestanian M, Bevec D, Willheim M, Agis H, Kapiotis S et al. The mast cell as site of tissue-type plasminogen activator expression and fibrinolysis. J Immunol 1999; 162: 1032–1041.
Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371: 2488–2498.
Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P . Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015; 125: 901–906.
Lassila M, Jandeleit-Dahm K, Seah KK, Smith CM, Calkin AC, Allen TJ et al. Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol 2005; 16: 363–373.
Chislock EM, Pendergast AM . Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS One 2013; 8: e85231.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369: 1783–1796.
Acknowledgements
We thank Sabine Cerny-Reiterer, Michael Kundi, Verena Suppan, Gabriele Stefanzl, Daniela Berger, Michael Gurbisz, Irina Mirkina, Sarah Vittori, Daniela Lener, Ursula Stanzl and Christoph Seger for excellent technical assistance. This study was supported in part by research funding from Novartis Oncology to PV and the Austrian Science Fund (FWF): F 4701-B20, F 4704-B20, F 5404-B20, and F 5412-B20.
Author contributions
EH, KAS, GE, IS, CK and JW performed experiments on cultured endothelial cells. KH, FG, UR and GSF performed proteomics and MS studies. WS, MT and RK performed experiments in mice. GH performed molecular studies. BJ performed studies on platelet adhesion and aggregation. SH, GHS, WRS and PV provided patients and analyzed the clinical and laboratory results. EH, DW, RK and PV drafted parts of the manuscript. EH and PV established the study concept and wrote the final paper-draft.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
EH received honoraria from Novartis. GHS received honoraria from Amgen, Astra Zeneca, Boehringer Ingelheim, BMS, Elli Lilly, Merck, Novo-Nordisk, Novartis, Pfizer and Sanofi-Aventis. DW received honoraria from Pfizer, BMS, Novartis, and Ariad and Research Grants from Pfizer and Ariad. RK received honoraria from Ariad and a Research Grant from Ariad. GH received honoraria form Novartis and Ariad. PV received honoraria from Novartis, Celgene, Pfizer, Deciphera, BMS, Ariad, and Incyte; and research grants from Novartis, Deciphera and Ariad. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Hadzijusufovic, E., Albrecht-Schgoer, K., Huber, K. et al. Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia 31, 2388–2397 (2017). https://doi.org/10.1038/leu.2017.245
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.245
This article is cited by
-
Nilotinib-induced alterations in endothelial cell function recapitulate clinical vascular phenotypes independent of ABL1
Scientific Reports (2024)
-
15 years Ludwig Boltzmann Institute for Hematology and Oncology (LBI HO): achievements and future perspectives
memo - Magazine of European Medical Oncology (2024)
-
Endothelial function measured by peripheral arterial tonometry in patients with chronic myeloid leukemia on tyrosine kinase inhibitor therapy: a pilot study
Cardio-Oncology (2023)
-
Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management
Signal Transduction and Targeted Therapy (2023)
-
Mitochondrial Dysfunction in Cardiotoxicity Induced by BCR-ABL1 Tyrosine Kinase Inhibitors -Underlying Mechanisms, Detection, Potential Therapies
Cardiovascular Toxicology (2023)