Abstract
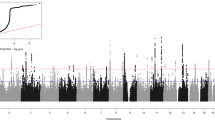
A genome-wide association study was carried out in 1020 case subjects with recurrent early-onset major depressive disorder (MDD) (onset before age 31) and 1636 control subjects screened to exclude lifetime MDD. Subjects were genotyped with the Affymetrix 6.0 platform. After extensive quality control procedures, 671 424 autosomal single nucleotide polymorphisms (SNPs) and 25 068 X chromosome SNPs with minor allele frequency greater than 1% were available for analysis. An additional 1 892 186 HapMap II SNPs were analyzed based on imputed genotypic data. Single-SNP logistic regression trend tests were computed, with correction for ancestry-informative principal component scores. No genome-wide significant evidence for association was observed, assuming that nominal P<5 × 10−8 approximates a 5% genome-wide significance threshold. The strongest evidence for association was observed on chromosome 18q22.1 (rs17077540, P=1.83 × 10−7) in a region that has produced some evidence for linkage to bipolar-I or -II disorder in several studies, within an mRNA detected in human brain tissue (BC053410) and approximately 75 kb upstream of DSEL. Comparing these results with those of a meta-analysis of three MDD GWAS data sets reported in a companion article, we note that among the strongest signals observed in the GenRED sample, the meta-analysis provided the greatest support (although not at a genome-wide significant level) for association of MDD to SNPs within SP4, a brain-specific transcription factor. Larger samples will be required to confirm the hypothesis of association between MDD (and particularly the recurrent early-onset subtype) and common SNPs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Pakriev S, Shlik J, Vasar V . Course of depression: findings from cross-sectional survey in rural Udmurtia. Nord J Psychiatry 2001; 55: 185–189.
Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML . Lifetime suicide risk in major depression: sex and age determinants. J Affect Disord 1999; 55: 171–178.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders,, 4th edn. American Psychiatric Press: Washington, DC, 1994.
Sullivan PF, Neale MC, Kendler KS . Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157: 1552–1562.
McGuffin P, Katz R, Watkins S, Rutherford J . A hospital-based twin register of the heritability of DSM-IV unipolar depression. Arch Gen Psychiatry 1996; 53: 129–136.
Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ . The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch Gen Psychiatry 1993; 50: 863–870.
Kendler KS, Kuhn JW, Prescott CA . Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004; 34: 1475–1482.
Zimmermann P, Bruckl T, Lieb R, Nocon A, Ising M, Beesdo K et al. The interplay of familial depression liability and adverse events in predicting the first onset of depression during a 10-year follow-up. Biol Psychiatry 2008; 63: 406–414.
Kendler KS, Gatz M, Gardner CO, Pedersen NL . Clinical indices of familial depression in the Swedish Twin Registry. Acta Psychiatr Scand 2007; 115: 214–220.
Levinson DF, Zubenko GS, Crowe RR, DePaulo RJ, Scheftner WS, Weissman MM et al. Genetics of recurrent early-onset depression (GenRED). Am J Med Genet B Neuropsychiatr Genet 2003; 119: 118–130.
Weissman MM, Wickramaratne P, Merikangas KR, Leckman JF, Prusoff BA, Caruso KA et al. Onset of major depression in early adulthood. Increased familial loading and specificity. Arch Gen Psychiatry 1984; 41: 1136–1143.
Kendler KS, Gardner CO, Neale MC, Prescott CA . Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med 2001; 31: 605–616.
Kendler KS, Gatz M, Gardner CO, Pedersen NL . A Swedish national twin study of lifetime major depression. Am J Psychiatry 2006; 163: 109–114.
Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O et al. Continuity and discontinuity of affective disorders and schizophrenia. Results of a controlled family study. Arch Gen Psychiatry 1993; 50: 871–883.
Holmans P, Weissman MM, Zubenko GS, Scheftner WA, Crowe RR, Depaulo Jr JR et al. Genetics of recurrent early-onset major depression (GenRED): final genome scan report. Am J Psychiatry 2007; 164: 248–258.
Levinson DF, Evgrafov OV, Knowles JA, Potash JB, Weissman MM, Scheftner WA et al. Genetics of recurrent early-onset major depression (GenRED): significant linkage on chromosome 15q25-q26 after fine mapping with single nucleotide polymorphism markers. Am J Psychiatry 2007; 164: 259–264.
Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM et al. Novel loci for major depression identified by genome-wide association study of STAR*D and meta-analysis of three studies. Mol Psychiatry, published online 29 December 2009; e-pub ahead of print.
Psychiatric GWAS Consortium Coordinating Committee, Cichon S, Craddock N, Daly M, Faraone SV, Gejman PV et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry 2009; 166: 540–556.
Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry 2008; 165: 497–506.
Nurnberger Jr JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51: 849–859.
Kessler RC, Andrews G, Mroczek D, Ustun TB, Wittchen H-U . The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF). Int J Methods Psychiatr Res 1998; 7: 171–185.
Korn JM, Kuruvilla FG, McCarroll SA, Wysoker A, Nemesh J, Cawley S et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet 2008; 40: 1253–1260.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D . Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909.
Huang L, Li Y, Singleton AB, Hardy JA, Abecasis G, Rosenberg NA et al. Genotype-imputation accuracy across worldwide human populations. Am J Hum Genet 2009; 84: 235–250.
Marchini J, Howie B, Myers S, McVean G, Donnelly P . A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007; 39: 906–913.
Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009; 41: 56–65.
Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341–1345.
Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008; 40: 161–169.
Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009; 41: 25–34.
Dudbridge F, Gusnanto A . Estimation of significance thresholds for genomewide association scans. Genet Epidemiol 2008; 32: 227–234.
Hoggart CJ, Clark TG, De Iorio M, Whittaker JC, Balding DJ . Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol 2008; 32: 179–185.
Pe’er I, Yelensky R, Altshuler D, Daly MJ . Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 2008; 32: 381–385.
Devlin B, Roeder K . Genomic control for association studies. Biometrics 1999; 55: 997–1004.
Taylor J, Tyekucheva S, King DC, Hardison RC, Miller W, Chiaromonte F . ESPERR: learning strong and weak signals in genomic sequence alignments to identify functional elements. Genome Res 2006; 16: 1596–1604.
Ferreira MAR, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40: 1056–1058.
Manolio TA, Brooks LD, Collins FS . A HapMap harvest of insights into the genetics of common disease. J Clin Invest 2008; 118: 1590–1605.
Psychiatric GWAS Consortium Coordinating Committee Cichon S, Craddock N, Daly M, Faraone SV, Gejman PV et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry 2009; 166: 540–556.
Psychiatric GWAS Consortium. A framework for interpreting genome-wide association studies of psychiatric disorders. Mol Psychiatry 2009; 14: 10–17.
Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D et al. Genomewide linkage scan for bipolar-disorder susceptibility loci among Ashkenazi Jewish families. Am J Hum Genet 2004; 75: 204–219.
McMahon FJ, Hopkins PJ, Xu J, McInnis MG, Shaw S, Cardon L et al. Linkage of bipolar affective disorder to chromosome 18 markers in a new pedigree series. Am J Hum Genet 1997; 61: 1397–1404.
McMahon FJ, Simpson SG, McInnis MG, Badner JA, MacKinnon DF, DePaulo JR . Linkage of bipolar disorder to chromosome 18q and the validity of bipolar II disorder. Arch Gen Psychiatry 2001; 58: 1025–1031.
Nwulia EA, Miao K, Zandi PP, Mackinnon DF, DePaulo Jr JR, McInnis MG . Genome-wide scan of bipolar II disorder. Bipolar Disord 2007; 9: 580–588.
Stine OC, Xu J, Koskela R, McMahon FJ, Gschwend M, Friddle C et al. Evidence for linkage of bipolar disorder to chromosome 18 with a parent-of-origin effect. Am J Hum Genet 1995; 57: 1384–1394.
Verheyen GR, Villafuerte SM, Del-Favero J, Souery D, Mendlewicz J, Van Broeckhoven C et al. Genetic refinement and physical mapping of a chromosome 18q candidate region for bipolar disorder. Eur J Hum Genet 1999; 7: 427–434.
McInnes LA, Escamilla MA, Service SK, Reus VI, Leon P, Silva S et al. A complete genome screen for genes predisposing to severe bipolar disorder in two Costa Rican pedigrees. Proc Natl Acad Sci U S A 1996; 93: 13060–13065.
McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet 2005; 77: 582–595.
Levinson DF . The genetics of depression: a review. Biol Psychiatry 2006; 60: 84–92.
Goossens D, Van Gestel S, Claes S, De Rijk P, Souery D, Massat I et al. A novel CpG-associated brain-expressed candidate gene for chromosome 18q-linked bipolar disorder. Mol Psychiatry 2003; 8: 83–89.
Krishnan V, Nestler EJ . The molecular neurobiology of depression. Nature 2008; 455: 894–902.
Supp DM, Witte DP, Branford WW, Smith EP, Potter SS . Sp4, a member of the sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Dev Biol 1996; 176: 284–299.
Suske G . The Sp-family of transcription factors. Gene 1999; 238: 291–300.
Zhou X, Long JM, Geyer MA, Masliah E, Kelsoe JR, Wynshaw-Boris A et al. Reduced expression of the Sp4 gene in mice causes deficits in sensorimotor gating and memory associated with hippocampal vacuolization. Mol Psychiatry 2004; 10: 393–406.
Safe S, Kim K . Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 2008; 41: 263–275.
Mao X, Moerman-Herzog AM, Wang W, Barger SW . Differential transcriptional control of the superoxide dismutase-2 kappaB element in neurons and astrocytes. J Biol Chem 2006; 281: 35863–35872.
Mao X, Yang SH, Simpkins JW, Barger SW . Glutamate receptor activation evokes calpain-mediated degradation of Sp3 and Sp4, the prominent Sp-family transcription factors in neurons. J Neurochem 2007; 100: 1300–1314.
Zhou X, Qyang Y, Kelsoe JR, Masliah E, Geyer MA . Impaired postnatal development of hippocampal dentate gyrus in Sp4 null mutant mice. Genes Brain Behav 2007; 6: 269–276.
Zhou X, Barrett TB, Kelsoe JR . Promoter variant in the GRK3 gene associated with bipolar disorder alters gene expression. Biol Psychiatry 2008; 64: 104–110.
Acknowledgements
The GenRED project is supported by grants from NIMH. We acknowledge the contributions of Dr George S Zubenko and Dr Wendy N Zubenko, Department of Psychiatry, University of Pittsburgh School of Medicine, to the GenRED I project. The NIMH Cell Repository at Rutgers University and the NIMH Center for Collaborative Genetic Studies on Mental Disorders made essential contributions to this project. Genotyping was carried out by the Broad Institute Center for Genotyping and Analysis with support from grant U54 RR020278 (which partially subsidized the genotyping of the GenRED cases) from the National Center for Research Resources. GWAS data for the GAIN-MDD data set were accessed by DFL through the Genetic Association Information Network (GAIN), through dbGaP accession number phs000020.v1.p1 (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000020.v2.p1); samples and associated phenotype data for Major Depression: Stage 1 Genome-Wide Association in Population-Based Samples were provided by P Sullivan. Data for Molecular Genetics of Schizophrenia (MGS) control subjects were used here by permission of the MGS project. Collection and quality control analyses of the control data set were supported by grants from NIMH and the National Alliance for Research on Schizophrenia and Depression. Genotyping of the controls was supported by grants from NIMH and by the Genetic Association Information Network (GAIN)(http://www.fnih.org/index.php?option=com_content&task=view&id=338&Itemid=454). Control data are available through dbGAP (http://www.ncbi.nlm.nih.gov/gap). We are grateful to Knowledge Networks Inc. (Menlo Park, CA, USA) for assistance in collecting the control data set. We express their profound appreciation to the families who participated in this project, and to the many clinicians who facilitated the referral of participants to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Shi, J., Potash, J., Knowles, J. et al. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry 16, 193–201 (2011). https://doi.org/10.1038/mp.2009.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2009.124
Keywords
This article is cited by
-
Genetics, epigenetics, and neurobiology of childhood-onset depression: an umbrella review
Molecular Psychiatry (2023)
-
Body mass index interacts with a genetic-risk score for depression increasing the risk of the disease in high-susceptibility individuals
Translational Psychiatry (2022)
-
Identification of putative causal loci in whole-genome sequencing data via knockoff statistics
Nature Communications (2021)
-
Whole genome sequencing of nearly isogenic WMI and WLI inbred rats identifies genes potentially involved in depression and stress reactivity
Scientific Reports (2021)
-
The role of rs242941, rs1876828, rs242939 and rs110402 polymorphisms of CRHR1 gene and the depression: systematic review and meta-analysis
Genes & Genomics (2021)