Abstract
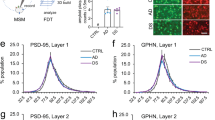
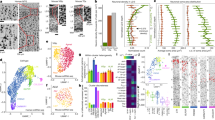
Cortical circuitry dysfunction in schizophrenia has been studied at many different levels of resolution, but not at the most basic unit of network organization—synaptic inputs. Multi-label electron or confocal light microscopy is required to examine specific types of synaptic inputs, and application of these methods to quantitatively study disease-related changes in human postmortem tissue has not been feasible for technical reasons. We recently developed a multi-label confocal light microscopic approach that makes possible the systematic identification and quantification of synaptic inputs, and of the relative levels of proteins localized to these inputs, in human postmortem tissue. We applied this approach to quantify parvalbumin basket cell (PVBC) inputs in area 9 of the dorsolateral prefrontal cortex from schizophrenia and matched comparison subjects. Tissue sections were triple-labeled for the 65 kD isoform of glutamic acid decarboxylase (GAD65), PV and the GABAA receptor α1 subunit. PVBC axonal boutons were defined as PV/GAD65 dual-labeled puncta, and PVBC inputs were defined as a PVBC bouton that overlapped a GABAA receptor α1 subunit punctum. The density of PVBC inputs was unchanged in subjects with schizophrenia, but levels of PV protein were lower in PVBC boutons. In concert with prior reports, these findings indicate that PVBC dysfunction in schizophrenia reflects molecular and not structural alterations in these cells and their axon terminals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Elvevag B, Goldberg TE . Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 2000; 14: 1–21.
Miller EK, Cohen JD . An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202.
Lewis DA, Gonzalez-Burgos G . Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacol Rev 2008; 33: 141–165.
Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003; 13: 1369–1374.
Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W . A new look at gamma? High- (>60 Hz) gamma-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol 2011; 105: 14–28.
Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ . Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci 2012; 32: 12411–12420.
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC . Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 2009; 66: 811–822.
Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS . Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010; 35: 2590–2599.
Cho RY, Konecky RO, Carter CS . Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA 2006; 103: 19878–19883.
Bartos M, Vida I, Jonas P . Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8: 45–56.
Melchitzky DS, Sesack SR, Lewis DA . Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: laminar, regional and target specificity of type I and type II synapses. J Comp Neurol 1999; 408: 11–22.
Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T . Segregation of axonal and somatic activity during fast network oscillations. Science 2012; 336: 1458–1461.
Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T . Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 2007; 27: 8184–8189.
Hajos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF . Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 2004; 24: 9127–9137.
Sohal VS, Zhang F, Yizhar O, Deisseroth K . Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009; 459: 698–702.
Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009; 459: 663–667.
Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 2007; 53: 591–604.
Gulyas AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 2010; 30: 15134–15145.
Klausberger T, Somogyi P . Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 2008; 321: 53–57.
Lewis DA, Curley AA, Glausier JR, Volk DW . Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35: 57–67.
Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA . Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 1994; 341: 95–116.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23: 6315–6326.
Lewis DA, Cruz DA, Melchitzky DS, Pierri JN . Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry 2001; 158: 1411–1422.
Woo T-U, Miller JL, Lewis DA . Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry 1997; 154: 1013–1015.
Beasley CL, Zhang ZJ, Patten I, Reynolds GP . Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 2002; 52: 708–715.
Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 2011; 168: 921–929.
Klausberger T, Roberts JD, Somogyi P . Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci 2002; 22: 2513–2521.
Nyíri G, Freund TF, Somogyi P . Input-dependent synaptic targeting of α2 subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 2001; 13: 428–442.
Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C et al. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatry Res 2010; 44: 673–681.
Beneyto M, Abbott A, Hashimoto T, Lewis DA . Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cereb Cortex 2011; 21: 999–1011.
Glausier JR, Lewis DA . Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology 2011; 36: 2103–2110.
Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA et al. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry 2008; 165: 479–489.
Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE Jr et al. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex 1995; 5: 550–560.
Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 2008; 13: 147–161.
Fish KN, Sweet RA, Lewis DA . Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex 2011; 21: 2450–2460.
Benke D, Cicin-Sain A, Mertens S, Mohler H . Immunochemical identification of the α1- and α3-subunits of the GABAA-receptor in rat brain. J Receptor Res 1991; 11: 407–424.
Schwaller B, Dick J, Dhoot G, Carroll S, Vrbova G, Nicotera P et al. Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am J Physiol 1999; 276: C395–C403.
Billinton N, Knight AW . Seeing the wood through the trees: a review of techniques for distinguishing green fluorescent protein from endogenous autofluorescence. Anal Biochem 2001; 291: 175–197.
Ridler TW, Calvard S . Picture thresholding using an iterative selection method, IEEE Trans. System Man Cybernetics 1978; 8: 630–632.
Kaufman DL, Houser CR, Tobin AJ . Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem 1991; 56: 720–723.
Mohler H . GABA(A) receptor diversity and pharmacology. Cell Tissue Res 2006; 326: 505–516.
Fish KN, Sweet RA, Deo AJ, Lewis DA . An automated segmentation methodology for quantifying immunoreactive puncta number and fluorescence intensity in tissue sections. Brain Res 2008; 1240: 62–72.
Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci 2007; 27: 11254–11262.
Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA . Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci 2013; 33: 8352–8358.
Lesh TA, Niendam TA, Minzenberg MJ, Carter CS . Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 2011; 36: 316–338.
Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M et al. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry 1999; 156: 1328–1335.
Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry 2010; 167: 160–169.
Carder RK, Leclerc SS, Hendry SHC . Regulation of calcium-binding protein immunoreactivity in GABA neurons of macaque primary visual cortex. Cereb Cortex 1996; 6: 271–287.
Jones EG . GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex 1993; 3: 361–372.
Melchitzky DS, Lewis DA . Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex 2003; 13: 452–460.
Glantz LA, Lewis DA . Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57: 65–73.
Volman V, Behrens MM, Sejnowski TJ . Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 2011; 31: 18137–18148.
Acknowledgements
The authors gratefully acknowledge Brad Rocco for his expertise and assistance with image processing, and Wasiq Sheikh for his imaging assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
David A Lewis currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2011–2013 served as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb and Concert Pharmaceuticals. Drs Glausier and Fish have no conflict of interest.
Rights and permissions
About this article
Cite this article
Glausier, J., Fish, K. & Lewis, D. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 19, 30–36 (2014). https://doi.org/10.1038/mp.2013.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.152
Keywords
This article is cited by
-
Altered Rbfox1-Vamp1 pathway and prefrontal cortical dysfunction in schizophrenia
Molecular Psychiatry (2024)
-
Effects of the mGlu2/3 receptor agonist LY379268 on two models of disturbed auditory evoked brain oscillations in mice
Translational Psychiatry (2023)
-
Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications
Molecular Psychiatry (2022)
-
Role of the NRG1/ErbB4 and PI3K/AKT/mTOR signaling pathways in the anti-psychotic effects of aripiprazole and sertindole in ketamine-induced schizophrenia-like behaviors in rats
Inflammopharmacology (2022)
-
Non-cell-autonomous OTX2 transcription factor regulates anxiety-related behavior in the mouse
Molecular Psychiatry (2021)