Abstract
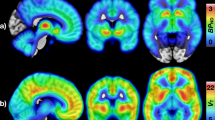
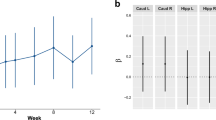
The obesity epidemic is believed to be driven by a food environment that promotes consumption of inexpensive, convenient, high-calorie, palatable foods. Individual differences in obesity susceptibility or resistance to weight loss may arise because of alterations in the neurocircuitry supporting food reward and eating habits. In particular, dopamine signaling in the ventromedial striatum is thought to encode food reward and motivation, whereas dopamine in the dorsal and lateral striatum orchestrates the development of eating habits. We measured striatal dopamine D2-like receptor binding potential (D2BP) using positron emission tomography with [18F]fallypride in 43 human subjects with body mass indices (BMI) ranging from 18 to 45 kg m−2. Opportunistic eating behavior and BMI were both positively associated with D2BP in the dorsal and lateral striatum, whereas BMI was negatively associated with D2BP in the ventromedial striatum. These results suggest that obese people have alterations in dopamine neurocircuitry that may increase their susceptibility to opportunistic overeating while at the same time making food intake less rewarding, less goal directed and more habitual. Whether or not the observed neurocircuitry alterations pre-existed or occurred as a result of obesity development, they may perpetuate obesity given the omnipresence of palatable foods and their associated cues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41380-022-01767-5
References
Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378: 804–814.
Bond MJ, McDowell AJ, Wilkinson JY . The measurement of dietary restraint, disinhibition and hunger: an examination of the factor structure of the Three Factor Eating Questionnaire (TFEQ). Int J Obes Relat Metab Disord 2001; 25: 900–906.
Bryant EJ, King NA, Blundell JE . Disinhibition: its effects on appetite and weight regulation. Obes Rev 2008; 9: 409–419.
Graybiel AM . Habits, rituals, and the evaluative brain. Annu Rev Neurosci 2008; 31: 359–387.
Wickens JR, Horvitz JC, Costa RM, Killcross S . Dopaminergic mechanisms in actions and habits. J Neurosci 2007; 27: 8181–8183.
Yin HH, Knowlton BJ . The role of the basal ganglia in habit formation. Nat Rev Neurosci 2006; 7: 464–476.
Yin HH, Ostlund SB, Balleine BW . Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci 2008; 28: 1437–1448.
Nelson A, Killcross S . Amphetamine exposure enhances habit formation. J Neurosci 2006; 26: 3805–3812.
Faure A, Haberland U, Conde F, El Massioui N . Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci 2005; 25: 2771–2780.
Murray JE, Dilleen R, Pelloux Y, Economidou D, Dalley JW, Belin D et al. Increased impulsivity retards the transition to dorsolateral striatal dopamine control of cocaine seeking. Biol Psychiatry 2014; 76: 15–22.
Yin HH, Knowlton BJ, Balleine BW . Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 2004; 19: 181–189.
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al. Brain dopamine and obesity. Lancet 2001; 357: 354–357.
Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG . The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010; 1350: 43–64.
Stunkard AJ, Messick S . The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Lammertsma AA, Hume SP . Simplified reference tissue model for PET receptor studies. NeuroImage 1996; 4: 153–158.
Berthoud HR . The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 2012; 71: 478–487.
Rangel A . Regulation of dietary choice by the decision-making circuitry. Nature Neurosci 2013; 16: 1717–1724.
Volkow ND, Wang GJ, Tomasi D, Baler RD . The addictive dimensionality of obesity. Biol Psychiatry 2013; 73: 811–818.
Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff-Guerrero A . Ventral striatum binding of a dopamine D receptor agonist but not antagonist predicts normal body mass index. Biol Psychiatry 2013. e-pub ahead of print 27 March 2013; doi:10.1016/j.biopsych.2013.02.017.
Eisenstein SA, Antenor-Dorsey JA, Gredysa DM, Koller JM, Bihun EC, Ranck SA et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[C]methyl)benperidol. Synapse 2013; 67: 748–756.
Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg 2010; 20: 369–374.
de Weijer BA, van de Geissen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res 2011; 1: 37.
Haltia LT, Rinne JO, Merisaari H, Maguire RP, Savontaus E, Helin S et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 2007; 61: 748–756.
Small DM, Jones-Gotman M, Dagher A . Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage 2003; 19: 1709–1715.
Elsinga PH, Hatano K, Ishiwata K . PET tracers for imaging of the dopaminergic system. Curr Med Chem 2006; 13: 2139–2153.
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 2000; 97: 8104–8109.
Morris ED, Yoder KK . Positron emission tomography displacement sensitivity: predicting binding potential change for positron emission tomography tracers based on their kinetic characteristics. J Cereb Blood Flow Metab 2007; 27: 606–617.
Alsio J, Olszewski PK, Norback AH, Gunnarsson ZE, Levine AS, Pickering C et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 2010; 171: 779–787.
Johnson PM, Kenny PJ . Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neurosci 2010; 13: 635–641.
Acknowledgements
We thank Kaiping Burrows, Valerie Darcy, Christine Hunter, John Ingeholm, Bernard V Miller, Monica Skarulis and Nora Volkow for their assistance with the study. This research was supported, in part, by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases and National Institute of Mental Health. WKS’ efforts were also partially supported by the NIMH K01MH096175-01 grant. The study was conducted under NIH Clinical Study Protocol 09-DK-0081 (ClinicalTrials.gov ID NCT00846040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Guo, J., Simmons, W., Herscovitch, P. et al. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry 19, 1078–1084 (2014). https://doi.org/10.1038/mp.2014.102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.102
This article is cited by
-
A literature review of dopamine in binge eating
Journal of Eating Disorders (2022)
-
Neuroimaging and modulation in obesity and diabetes research: 10th anniversary meeting
International Journal of Obesity (2022)
-
Sex differences in methylphenidate-induced dopamine increases in ventral striatum
Molecular Psychiatry (2022)
-
Contrasting dorsal caudate functional connectivity patterns between frontal and temporal cortex with BMI increase: link to cognitive flexibility
International Journal of Obesity (2021)
-
The associations of BMI with mean diffusivity of basal ganglia among young adults with mild obesity and without obesity
Scientific Reports (2020)