Abstract
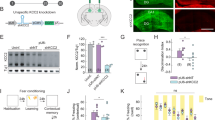
Overexpression in humans of KCNH2-3.1, which encodes a primate-specific and brain-selective isoform of the human ether-a-go-go-related potassium channel, is associated with impaired cognition, inefficient neural processing and schizophrenia. Here, we describe a new mouse model that incorporates the KCNH2-3.1 molecular phenotype. KCNH2-3.1 transgenic mice are viable and display normal sensorimotor behaviors. However, they show alterations in neuronal structure and microcircuit function in the hippocampus and prefrontal cortex, areas affected in schizophrenia. Specifically, in slice preparations from the CA1 region of the hippocampus, KCNH2-3.1 transgenic mice have fewer mature dendrites and impaired theta burst stimulation long-term potentiation. Abnormal neuronal firing patterns characteristic of the fast deactivation kinetics of the KCNH2-3.1 isoform were also observed in prefrontal cortex. Transgenic mice showed significant deficits in a hippocampal-dependent object location task and a prefrontal cortex-dependent T-maze working memory task. Interestingly, the hippocampal-dependent alterations were not present in juvenile transgenic mice, suggesting a developmental trajectory to the phenotype. Suppressing KCNH2-3.1 expression in adult mice rescues both the behavioral and physiological phenotypes. These data provide insight into the mechanism of association of KCNH2-3.1 with variation in human cognition and neuronal physiology and may explain its role in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med 2009; 15: 509–518.
Hashimoto R, Ohi K, Yasuda Y, Fukumoto M, Yamamori H, Kamino K et al. The KCNH2 gene is associated with neurocognition and the risk of schizophrenia. World J Biol Psychiatry 2013; 14: 114–120.
Atalar F, Acuner TT, Cine N, Oncu F, Yesilbursa D, Ozbek U et al. Two four-marker haplotypes on 7q36.1 region indicate that the potassium channel gene HERG1 (KCNH2, Kv11.1) is related to schizophrenia: a case control study. Behav Brain Funct 2010; 6: 27.
Trudeau MC, Warmke JW, Ganetzky B, Robertson GA . HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 1995; 269: 92–95.
Trudeau MC, Warmke JW, Ganetzky B, Robertson GA . HERG sequence correction. Science 1996; 272: 1087.
Pessia M, Servettini I, Panichi R, Guasti L, Grassi S, Arcangeli A et al. ERG voltage-gated K+ channels regulate excitability and discharge dynamics of the medial vestibular nucleus neurones. J Physiol 2008; 586: 4877–4890.
Ji H, Tucker KR, Putzier I, Huertas MA, Horn JP, Canavier CC et al. Functional characterization of ether-à-go-go-related gene potassium channels in midbrain dopamine neurons - implications for a role in depolarization block. Eur J Neurosci 2012; 36: 2906–2916.
Fano S, Çalışkan G, Heinemann U . Differential effects of blockade of ERG channels on gamma oscillations and excitability in rat hippocampal slices. Eur J Neurosci 2012; 36: 3628–3635.
Keefe RS, Harvey PD . Cognitive impairment in schizophrenia. Handb Exp Pharmacol 2012; 213: 11–37.
Green MF, Kern RS, Braff DL, Mintz J . Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull 2000; 26: 119–136.
Mowry BJ, Gratten J . The emerging spectrum of allelic variation in schizophrenia: current evidence and strategies for the identification and functional characterization of common and rare variants. Mol Psychiatry 2013; 18: 38–52.
Svrakic DM, Zorumski CF, Svrakic NM, Zwir I, Cloninger CR . Risk architecture of schizophrenia: the role of epigenetics. Curr Opin Psychiatry 2013; 26: 188–195.
Apud JA, Zhang F, Decot H, Bigos KL, Weinberger DR . Genetic variation in KCNH2 associated with expression in the brain of a unique hERG isoform modulates treatment response in patients with schizophrenia. Am J Psychiatry 2012; 169: 725–734.
Barker GR, Bird F, Alexander V, Warburton EC . Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 2007; 27: 2948–2957.
Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci 2008; 28: 8709–8723.
Barker GR, Warburton EC . When is the hippocampus involved in recognition memory? J Neurosci 2011; 31: 10721–10731.
Shepard PD, Canavier CC, Levitan ES . Ether-a-go-go-related gene potassium channels: what's all the buzz about? Schizophr Bull 2007; 33: 1263–1269.
Aultman JM, Moghaddam B . Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001; 153: 353–364.
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006; 49: 603–615.
Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry 2012; 17: 85–98.
Weinberger DR . Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44: 660–669.
Pratt J, Winchester C, Dawson N, Morris B . Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat Rev Drug Discov 2012; 11: 560–579.
Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ . Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev 2012; 36: 1342–1356.
Adriano F, Caltagirone C, Spalletta G . Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist 2012; 18: 180–200.
Bogerts B, Meertz E, Schönfeldt-Bausch R . Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42: 784–791.
Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K . Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain 2013; 136: 804–814.
Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Zoltick B et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry 2008; 63: 475–483.
Owens SF, Picchioni MM, Rijsdijk FV, Stahl D, Vassos E, Rodger AK et al. Genetic overlap between episodic memory deficits and schizophrenia: results from the Maudsley Twin Study. Psychol Med 2011; 41: 521–532.
Hanlon FM, Weisend MP, Hamilton DA, Jones AP, Thoma RJ, Huang M et al. Impairment on the hippocampal-dependent virtual Morris water task in schizophrenia. Schizophr Res 2006; 87: 67–80.
Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 2006; 9: 381–388.
Mumby DG, Pinel JP . Rhinal cortex lesions and object recognition in rats. Behav Neurosci 1994; 108: 11–18.
Ennaceur A, Neave N, Aggleton JP . Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res 1996; 80: 9–25.
Asrar S, Kaneko K, Takao K, Negandhi J, Matsui M, Shibasaki K et al. DIP/WISH deficiency enhances synaptic function and performance in the Barnes maze. Mol Brain 2011; 4: 39.
Huerta PT, Sun LD, Wilson MA, Tonegawa S . Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 2000; 25: 473–480.
Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM et al. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 2011; 31: 6392–6397.
Lipska BK, Weinberger DR . To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology 2000; 23: 223–239.
Lee H, Dvorak D, Kao HY, Duffy Á, Scharfman HE, Fenton AA . Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron 2012; 75: 714–724.
Lodge DJ, Grace AA . Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res 2009; 204: 306–312.
Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R . Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci 2012; 35: 1605–1612.
Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 2001; 50: 825–844.
Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M et al. Neuronal basis of age-related working memory decline. Nature 2011; 476: 210–213.
Artchakov D, Tikhonravov D, Ma Y, Neuvonen T, Linnankoski I, Carlson S . Distracters impair and create working memory-related neuronal activity in the prefrontal cortex. Cereb Cortex 2009; 19: 2680–2689.
Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA 2010; 107: 1211–1216.
Heide J, Mann SA, Vandenberg JI . The schizophrenia-associated Kv11.1-3.1 isoform results in reduced current accumulation during repetitive brief depolarizations. PLOS One 2012; 7: e45624.
Cardoso-Cruz H, Lima D, Galhardo V . Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci 2013; 33: 2465–2480.
Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003; 13: 1369–1374.
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J . Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci 1998; 18: 4244–4254.
Papaleo F, Lipska BK, Weinberger DR . Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology 2012; 62: 1204–1220.
Owen MJ . Implications of genetic findings for understanding schizophrenia. Schizophr Bull 2012; 38: 904–907.
Acknowledgements
This work was funded by the Intramural Research Program of the National Institute of Mental Health to the Weinberger Lab, US National Institute of Mental Health grant R01MH101102 and the Lieber Institute for Brain Development.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Weinberger is an inventor on an NIH-held patent ‘Schizophrenia related isoform of KCNH2 and development of antipsychotic drugs’ (US patent no. 8101380). The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Carr, G., Chen, J., Yang, F. et al. KCNH2-3.1 expression impairs cognition and alters neuronal function in a model of molecular pathology associated with schizophrenia. Mol Psychiatry 21, 1517–1526 (2016). https://doi.org/10.1038/mp.2015.219
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.219
This article is cited by
-
Decreased CNNM2 expression in prefrontal cortex affects sensorimotor gating function, cognition, dendritic spine morphogenesis and risk of schizophrenia
Neuropsychopharmacology (2024)
-
An alternative splicing hypothesis for neuropathology of schizophrenia: evidence from studies on historical candidate genes and multi-omics data
Molecular Psychiatry (2022)
-
Analysis of KCNH2 and CACNA1C schizophrenia risk genes on EEG functional network modulation during an auditory odd-ball task
European Archives of Psychiatry and Clinical Neuroscience (2020)
-
Molecular mechanisms underlying noncoding risk variations in psychiatric genetic studies
Molecular Psychiatry (2017)