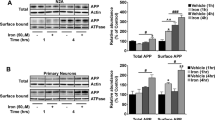
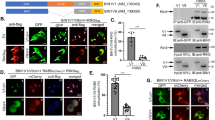
Abstract
Neuropathological hallmarks of Alzheimer's disease are neurofibrillary tangles composed of tau and neuritic plaques comprising amyloid-β peptides (Aβ) derived from amyloid precursor protein (APP), but their exact relationship remains elusive1,2,3. Phosphorylation of tau and APP on certain serine or threonine residues preceding proline affects tangle formation and Aβ production in vitro3,4,5. Phosphorylated Ser/Thr-Pro motifs in peptides can exist in cis or trans conformations, the conversion of which is catalysed by the Pin1 prolyl isomerase6,7. Pin1 has been proposed to regulate protein function by accelerating conformational changes7,8,9,10, but such activity has never been visualized and the biological and pathological significance of Pin1 substrate conformations is unknown7. Notably, Pin1 is downregulated and/or inhibited by oxidation in Alzheimer's disease neurons, Pin1 knockout causes tauopathy and neurodegeneration8,9,11,12, and Pin1 promoter polymorphisms appear to associate with reduced Pin1 levels and increased risk for late-onset Alzheimer's disease13,14. However, the role of Pin1 in APP processing and Aβ production is unknown. Here we show that Pin1 has profound effects on APP processing and Aβ production. We find that Pin1 binds to the phosphorylated Thr 668-Pro motif in APP and accelerates its isomerization by over 1,000-fold, regulating the APP intracellular domain between two conformations, as visualized by NMR. Whereas Pin1 overexpression reduces Aβ secretion from cell cultures, knockout of Pin1 increases its secretion. Pin1 knockout alone or in combination with overexpression of mutant APP in mice increases amyloidogenic APP processing and selectively elevates insoluble Aβ42 (a major toxic species) in brains in an age-dependent manner, with Aβ42 being prominently localized to multivesicular bodies of neurons, as shown in Alzheimer's disease before plaque pathology15. Thus, Pin1-catalysed prolyl isomerization is a novel mechanism to regulate APP processing and Aβ production, and its deregulation may link both tangle and plaque pathologies. These findings provide new insight into the pathogenesis and treatment of Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
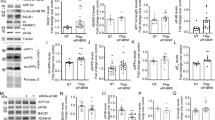
References
Mattson, M. P. Pathways towards and away from Alzheimer's disease. Nature 430, 631–639 (2004)
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002)
Lee, V. M., Goedert, M. & Trojanowski, J. Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 (2001)
Lu, K. P., Liou, Y. C. & Vincent, I. Proline-directed phosphorylation and isomerization in mitotic regulation and in Alzheimer's disease. Bioessays 25, 174–181 (2003)
Lee, M. S. et al. APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 163, 83–95 (2003)
Yaffe, M. B. et al. Sequence-specific and phosphorylation-dependent proline isomerization: A potential mitotic regulatory mechanism. Science 278, 1957–1960 (1997)
Lu, K. P. Pinning down cell signaling, cancer and Alzheimer's disease. Trends Biochem. Sci. 29, 200–209 (2004)
Lu, P. J., Wulf, G., Zhou, X. Z., Davies, P. & Lu, K. P. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399, 784–788 (1999)
Zhou, X. Z. et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell 6, 873–883 (2000)
Stukenberg, P. T. & Kirschner, M. W. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol. Cell 7, 1071–1083 (2001)
Liou, Y.-C. et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424, 556–561 (2003)
Sultana, R. et al. Oxidative modification and down-regulation of Pin1 in Alzheimer's disease hippocampus: A redox proteomics analysis. Neurobiol. Aging ahead of print publication, 9 June 2005 (doi:10.1016/j.neurobiolaging.2005.05.005).
Segat, L. et al. Pin1 promoter polymorphisms are associated with Alzheimer's disease. Neurobiol. Aging ahead of print publication, 26 December 2005 (doi:10.1016/j.neurobiolaging.2005.11.009).
Wijsman, E. M. et al. Evidence for a novel late-onset Alzheimer disease locus on chromosome 19p13.2. Am. J. Hum. Genet. 75, 398–409 (2004)
Takahashi, R. H. et al. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161, 1869–1879 (2002)
Ramelot, T. A. & Nicholson, L. K. Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J. Mol. Biol. 307, 871–884 (2001)
Ramelot, T. A., Gentile, L. N. & Nicholson, L. K. Transient structure of the amyloid precursor protein cytoplasmic tail indicates preordering of structure for binding to cytosolic factors. Biochemistry 39, 2714–2725 (2000)
Suzuki, T. et al. Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. EMBO J. 13, 1114–1122 (1994)
Lu, P. J., Zhou, X. Z., Shen, M. & Lu, K. P. A function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283, 1325–1328 (1999)
Ernst, R. R., Bodenhausen, G. & Wokaun, A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions (Claredon, Oxford, 1987)
Schutkowski, M. et al. Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition. Biochemistry 37, 5566–5575 (1998)
Wintjens, R. et al. 1H NMR study on the binding of Pin1 Trp-Trp domain with phosphothreonine peptides. J. Biol. Chem. 276, 25150–25156 (2001)
Wulf, G., Garg, P., Liou, Y. C., Iglehart, D. & Lu, K. P. Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. EMBO J. 23, 3397–3407 (2004)
Pastorino, L. et al. BACE (β-secretase) modulates the processing of APLP2 in vivo. Mol. Cell. Neurosci. 25, 642–649 (2004)
Citron, M. et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Med. 3, 67–72 (1997)
Scheuner, D. et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nature Med. 2, 864–870 (1996)
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996)
Duff, K. et al. Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713 (1996)
Borchelt, D. R. et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron 17, 1005–1013 (1996)
Suizu, F., Ryo, A., Wulf, G., Lim, J. & Lu, K. P. Pin1 regulates centrosome duplication and its overexpression induces centrosome amplification, chromosome instability and oncogenesis. Mol. Cell. Biol. 26, 1463–1479 (2006)
Acknowledgements
We thank D. Selkoe for advice, B. Arosio and G. Annoni for sharing results on Pin1 genetic analysis in Alzheimer's disease before publication, D. Selkoe, D. Schenk, P. Seubert, D. Goldgaber, E. Koo, J. Wang and H. Xu for reagents, and J. Sears, H. Yi and M. Wotkowicz for technical assistance. J.L. is a Fellow of the Human Frontier Research Program; G.W. is a NIH Mentored Clinician Scientist. This study was supported by a Taiwan NSC grant to P.J.L., an NSF grant to L.K.N. and NIH grants to X.L., W.X., X.Z.Z. and K.P.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods, Supplementary Figures 1–9 and additional references. (PDF 2019 kb)
Supplementary Data
This file contains a list of genes or proteins used in this study. (DOC 19 kb)
Rights and permissions
About this article
Cite this article
Pastorino, L., Sun, A., Lu, PJ. et al. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-β production. Nature 440, 528–534 (2006). https://doi.org/10.1038/nature04543
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04543
This article is cited by
-
Death-associated protein kinase 1 as a therapeutic target for Alzheimer's disease
Translational Neurodegeneration (2024)
-
Long-term normalization of calcineurin activity in model mice rescues Pin1 and attenuates Alzheimer’s phenotypes without blocking peripheral T cell IL-2 response
Alzheimer's Research & Therapy (2023)
-
The US9-Derived Protein gPTB9TM Modulates APP Processing Without Targeting Secretase Activities
Molecular Neurobiology (2023)
-
Spinal Cord Injury Causes Prominent Tau Pathology Associated with Brain Post-Injury Sequela
Molecular Neurobiology (2022)
-
The Role of Mitochondrial Quality Control in Cognitive Dysfunction in Diabetes
Neurochemical Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.