Abstract
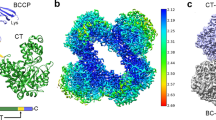
Propionyl-coenzyme A carboxylase (PCC), a mitochondrial biotin-dependent enzyme, is essential for the catabolism of the amino acids Thr, Val, Ile and Met, cholesterol and fatty acids with an odd number of carbon atoms. Deficiencies in PCC activity in humans are linked to the disease propionic acidaemia, an autosomal recessive disorder that can be fatal in infants1,2,3,4. The holoenzyme of PCC is an α6β6 dodecamer, with a molecular mass of 750 kDa. The α-subunit contains the biotin carboxylase (BC) and biotin carboxyl carrier protein (BCCP) domains, whereas the β-subunit supplies the carboxyltransferase (CT) activity. Here we report the crystal structure at 3.2-Å resolution of a bacterial PCC α6β6 holoenzyme as well as cryo-electron microscopy (cryo-EM) reconstruction at 15-Å resolution demonstrating a similar structure for human PCC. The structure defines the overall architecture of PCC and reveals unexpectedly that the α-subunits are arranged as monomers in the holoenzyme, decorating a central β6 hexamer. A hitherto unrecognized domain in the α-subunit, formed by residues between the BC and BCCP domains, is crucial for interactions with the β-subunit. We have named it the BT domain. The structure reveals for the first time the relative positions of the BC and CT active sites in the holoenzyme. They are separated by approximately 55 Å, indicating that the entire BCCP domain must translocate during catalysis. The BCCP domain is located in the active site of the β-subunit in the current structure, providing insight for its involvement in the CT reaction. The structural information establishes a molecular basis for understanding the large collection of disease-causing mutations in PCC and is relevant for the holoenzymes of other biotin-dependent carboxylases, including 3-methylcrotonyl-CoA carboxylase (MCC)5,6,7 and eukaryotic acetyl-CoA carboxylase (ACC)8,9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Desviat, L. R. et al. Propionic acidemia: mutation update and functional and structural effects of the variant alleles. Mol. Genet. Metab. 83, 28–37 (2004)
Rodriguez-Pombo, P. et al. Towards a model to explain the intragenic complementation in the heteromultimeric protein propionyl-CoA carboxylase. Biochim. Biophys. Acta 1740, 489–498 (2005)
Deodato, F., Boenzi, S., Santorelli, F. M. & Dionisi-Vici, C. Methylmalonic and propionic aciduria. Am. J. Med. Genet. C. Semin. Med. Genet. 142, 104–112 (2006)
Desviat, L. R. et al. New splicing mutations in propionic acidemia. J. Hum. Genet. 51, 992–997 (2006)
Desviat, L. R. et al. Functional analysis of MCCA and MCCB mutations causing methylcrotonylglycinuria. Mol. Genet. Metab. 80, 315–320 (2003)
Stadler, S. C. et al. Newborn screening for 3-methylcrotonyl-CoA carboxylase deficiency: population heterogeneity of MCCA and MCCB mutations and impact on risk assessment. Hum. Mutat. 27, 748–759 (2006)
Stucki, M., Suormala, T., Fowler, B., Valle, D. & Baumgartner, M. R. Cryptic exon activation by disruption of exon splice enhancer. Novel mechanism causing 3-methylcrotonyl-CoA carboxylase deficiency. J. Biol. Chem. 284, 28953–28957 (2009)
Wakil, S. J., Stoops, J. K. & Joshi, V. C. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 52, 537–579 (1983)
Tong, L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 62, 1784–1803 (2005)
Waldrop, G. L., Rayment, I. & Holden, H. M. Three-dimensional structure of the biotin carboxylase subunit of acetyl-CoA carboxylase. Biochem. 33, 10249–10256 (1994)
Cronan, J. E., Jr & Waldrop, G. L. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 41, 407–435 (2002)
Zhang, H., Yang, Z., Shen, Y. & Tong, L. Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase. Science 299, 2064–2067 (2003)
Holm, L., Kaariainen, S., Rosenstrom, P. & Schenkel, A. Searching protein structure databases with DaliLite v.3. Bioinformatics 24, 2780–2781 (2008)
Xiang, S. & Tong, L. Crystal structures of human and Staphylococcus aureus pyruvate carboxylase and molecular insights into the carboxyltransfer reaction. Nature Struct. Mol. Biol. 15, 295–302 (2008)
Janiyani, K., Bordelon, T., Waldrop, G. L. & Cronan, J. E., Jr Function of Escherichia coli biotin carboxylase requires catalytic activity of both subunits of the homodimer. J. Biol. Chem. 276, 29864–29870 (2001)
Shen, Y., Chou, C.-Y., Chang, G.-G. & Tong, L. Is dimerization required for the catalytic activity of bacterial biotin carboxylase? Mol. Cell 22, 807–818 (2006)
St. Maurice, M. et al. Domain architecture of pyruvate carboxylase, a biotin-dependent multifunctional enzyme. Science 317, 1076–1079 (2007)
Chou, C.-Y., Yu, L. P. C. & Tong, L. Crystal structure of biotin carboxylase in complex with substrates and implications for its catalytic mechanism. J. Biol. Chem. 284, 11690–11697 (2009)
Diacovich, L. et al. Crystal structure of the b-subunit of acyl-CoA carboxylase: structure-based engineering of substrate specificity. Biochem. 43, 14027–14036 (2004)
Lin, T. W. et al. Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 3072–3077 (2006)
Hall, P. R. et al. Transcarboxylase 12S crystal structure: hexamer assembly and substrate binding to a multienzyme core. EMBO J. 22, 2334–2347 (2003)
Bilder, P. et al. The structure of the carboxyltransferase component of acetyl-CoA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochem. 45, 1712–1722 (2006)
Wendt, K. S., Schall, I., Huber, R., Buckel, W. & Jacob, U. Crystal structure of the carboxyltransferase subunit of the bacterial sodium ion pump glutaconyl-coenzyme A decarboxylase. EMBO J. 22, 3493–3502 (2003)
Sloane, V. & Waldrop, G. L. Kinetic characterization of mutations found in propionic acidemia and methylcrotonylglycinuria. J. Biol. Chem. 279, 15772–15778 (2004)
Jiang, H., Rao, K. S., Yee, V. C. & Kraus, J. P. Characterization of four variant forms of human propionyl-CoA carboxylase expressed in Escherichia coli. J. Biol. Chem. 280, 27719–27727 (2005)
Muro, S. et al. Effect of PCCB gene mutations on the heteromeric and homomeric assembly of propionyl-CoA carboxylase. Mol. Genet. Metab. 74, 476–483 (2001)
Perez-Cerda, C. et al. Functional analysis of PCCB mutations causing propionic acidemia based on expression studies in deficient human skin fibroblasts. Biochim. Biophys. Acta 1638, 43–49 (2003)
Shen, Y., Volrath, S. L., Weatherly, S. C., Elich, T. D. & Tong, L. A mechanism for the potent inhibition of eukaryotic acetyl-coenzyme A carboxylase by soraphen A, a macrocyclic polyketide natural product. Mol. Cell 16, 881–891 (2004)
Weatherly, S. C., Volrath, S. L. & Elich, T. D. Expression and characterization of recombinant fungal acetyl-CoA carboxylase and isolation of a soraphen-binding domain. Biochem. J. 380, 105–110 (2004)
Pettersen, E. F. et al. UCSF Chimera – a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007)
Thoden, J. B., Blanchard, C. Z., Holden, H. M. & Waldrop, G. L. Movement of the biotin carboxylase B-domain as a result of ATP binding. J. Biol. Chem. 275, 16183–16190 (2000)
Diacovich, L. et al. Crystal structure of the β-subunit of acyl-CoA carboxylase: structure-based engineering of substrate specificity. Biochemistry 43, 14027–14036 (2004)
Xiang, S. & Tong, L. Crystal structures of human and Staphylococcus aureus pyruvate carboxylase and molecular insights into the carboxyltransfer reaction. Nature Struct. Mol. Biol. 15, 295–302 (2008)
CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography & NMR System: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Jogl, G., Tao, X., Xu, Y. & Tong, L. COMO: a program for combined molecular replacement. Acta Crystallogr. D 57, 1127–1134 (2001)
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999)
Pettersen, E. F. et al. UCSF Chimera – a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Blanchard, C. Z., Lee, Y. M., Frantom, P. A. & Waldrop, G. L. Mutations at four active site residues of biotin carboxylase abolish substrate-induced synergism by biotin. Biochemistry 38, 3393–3400 (1999)
Acknowledgements
We thank N. Whalen and H. Robinson for access to the X29A beamline at the National Synchrotron Light Source; J. Schwanof and R. Abramowitz for access to the X4A beamline; M. Sampat for help during the initial stages of the project; and W.W. Cleland for discussions. This research was supported in part by National Institutes of Health grants DK067238 (to L.T.), GM071940 and AI069015 (to Z.H.Z.). C.S.H. was also supported by a National Institutes of Health training program in molecular biophysics (GM08281).
Author information
Authors and Affiliations
Contributions
C.S.H., K.S.-B., Y.S. and B.D. performed the experiments, analysed the data and commented on the manuscript. L.T. and Z.H.Z. designed and performed the experiments, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Tables 1-4, References and Supplementary Figures 1-17 with legends. (PDF 7349 kb)
Rights and permissions
About this article
Cite this article
Huang, C., Sadre-Bazzaz, K., Shen, Y. et al. Crystal structure of the α6β6 holoenzyme of propionyl-coenzyme A carboxylase. Nature 466, 1001–1005 (2010). https://doi.org/10.1038/nature09302
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09302
This article is cited by
-
CryoEM structural exploration of catalytically active enzyme pyruvate carboxylase
Nature Communications (2022)
-
MCCC2 promotes HCC development by supporting leucine oncogenic function
Cancer Cell International (2021)
-
Biochemical and structural characterization of the BioZ enzyme engaged in bacterial biotin synthesis pathway
Nature Communications (2021)
-
A novel delins (c.773_819+47delinsAA) mutation of the PCCA gene associated with neonatal-onset propionic acidemia: a case report
BMC Medical Genetics (2020)
-
Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.