Abstract
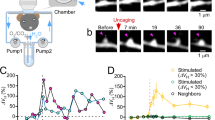
The individual functional properties and spatial arrangement of afferent synaptic inputs on dendrites have a critical role in the processing of information by neurons in the mammalian brain1,2,3,4. Although recent work has identified visually-evoked local dendritic calcium signals in the rodent visual cortex5, sensory-evoked signalling on the level of dendritic spines, corresponding to individual afferent excitatory synapses, remains unexplored6. Here we used a new variant of high-resolution two-photon imaging7 to detect sensory-evoked calcium transients in single dendritic spines of mouse cortical neurons in vivo. Calcium signals evoked by sound stimulation required the activation of NMDA (N-methyl-D-aspartate) receptors. Active spines are widely distributed on basal and apical dendrites and pure-tone stimulation at different frequencies revealed both narrowly and widely tuned spines. Notably, spines tuned for different frequencies were highly interspersed on the same dendrites: even neighbouring spines were mostly tuned to different frequencies. Thus, our results demonstrate that NMDA-receptor-dependent single-spine synaptic inputs to the same dendrite are highly heterogeneous. Furthermore, our study opens the way for in vivo mapping of functionally defined afferent sensory inputs with single-synapse resolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
London, M. & Häusser, M. Dendritic computation. Annu. Rev. Neurosci. 28, 503–532 (2005)
Cash, S. & Yuste, R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron 22, 383–394 (1999)
Sabatini, B. L., Maravall, M. & Svoboda, K. Ca2+ signaling in dendritic spines. Curr. Opin. Neurobiol. 11, 349–356 (2001)
Yuste, R., Majewska, A. & Holthoff, K. From form to function: calcium compartmentalization in dendritic spines. Nature Neurosci. 3, 653–659 (2000)
Jia, H., Rochefort, N. L., Chen, X. & Konnerth, A. Dendritic organization of sensory input to cortical neurons in vivo . Nature 464, 1307–1312 (2010)
Branco, T. & Häusser, M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr. Opin. Neurobiol. 20, 494–502 (2010)
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990)
Donnert, G., Eggeling, C. & Hell, S. W. Major signal increase in fluorescence microscopy through dark-state relaxation. Nature Methods 4, 81–86 (2007)
Ji, N., Magee, J. C. & Betzig, E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nature Methods 5, 197–202 (2008)
Kitamura, K., Judkewitz, B., Kano, M., Denk, W. & Häusser, M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo . Nature Methods 5, 61–67 (2008)
Jia, H., Rochefort, N. L., Chen, X. & Konnerth, A. In vivo two-photon imaging of sensory-evoked dendritic calcium signals in cortical neurons. Nature Protocols 6, 28–35 (2011)
Chadderton, P., Agapiou, J. P., McAlpine, D. & Margrie, T. W. The synaptic representation of sound source location in auditory cortex. J. Neurosci. 29, 14127–14135 (2009)
Linden, J. F., Liu, R. C., Sahani, M., Schreiner, C. E. & Merzenich, M. M. Spectrotemporal structure of receptive fields in areas AI and AAF of mouse auditory cortex. J. Neurophysiol. 90, 2660–2675 (2003)
Scholl, B., Gao, X. & Wehr, M. Nonoverlapping sets of synapses drive on responses and off responses in auditory cortex. Neuron 65, 412–421 (2010)
Waters, J., Larkum, M., Sakmann, B. & Helmchen, F. Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo . J. Neurosci. 23, 8558–8567 (2003)
Yuste, R. & Denk, W. Dendritic spines as basic functional units of neuronal integration. Nature 375, 682–684 (1995)
Kovalchuk, Y., Eilers, J., Lisman, J. & Konnerth, A. NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J. Neurosci. 20, 1791–1799 (2000)
Mainen, Z. F., Malinow, R. & Svoboda, K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature 399, 151–155 (1999)
Noguchi, J., Matsuzaki, M., Ellis-Davies, G. C. & Kasai, H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46, 609–622 (2005)
Berretta, N. & Jones, R. S. Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75, 339–344 (1996)
Wong, E. H. et al. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc. Natl Acad. Sci. USA 83, 7104–7108 (1986)
Bloodgood, B. L., Giessel, A. J. & Sabatini, B. L. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biol. 7, e1000190 (2009)
Bandyopadhyay, S., Shamma, S. A. & Kanold, P. O. Dichotomy of functional organization in the mouse auditory cortex. Nature Neurosci. 13, 361–368 (2010)
Rothschild, G., Nelken, I. & Mizrahi, A. Functional organization and population dynamics in the mouse primary auditory cortex. Nature Neurosci. 13, 353–360 (2010)
Wu, G. K., Li, P., Tao, H. W. & Zhang, L. I. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron 52, 705–715 (2006)
Larkum, M. E. & Nevian, T. Synaptic clustering by dendritic signalling mechanisms. Curr. Opin. Neurobiol. 18, 321–331 (2008)
Holthoff, K., Kovalchuk, Y., Yuste, R. & Konnerth, A. Single-shock LTD by local dendritic spikes in pyramidal neurons of mouse visual cortex. J. Physiol. 560, 27–36 (2004)
Polsky, A., Mel, B. W. & Schiller, J. Computational subunits in thin dendrites of pyramidal cells. Nature Neurosci. 7, 621–627 (2004)
Schiller, J., Major, G., Koester, H. J. & Schiller, Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature 404, 285–289 (2000)
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl Acad. Sci. USA 100, 7319–7324 (2003)
Franklin, K. & Paxinos, G. The Mouse Brain In Stereotaxic Coordinates. (Academic, 2001)
Busche, M. A. et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321, 1686–1689 (2008)
de Kock, C. P. & Sakmann, B. High frequency action potential bursts (≥100 Hz) in L2/3 and L5B thick tufted neurons in anaesthetized and awake rat primary somatosensory cortex. J. Physiol. 586, 3353–3364 (2008)
Svoboda, K., Helmchen, F., Denk, W. & Tank, D. W. Spread of dendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex in vivo . Nature Neurosci. 2, 65–73 (1999)
Lechleiter, J. D., Lin, D. T. & Sieneart, I. Multi-photon laser scanning microscopy using an acoustic optical deflector. Biophys. J. 83, 2292–2299 (2002)
Roorda, R. D., Hohl, T. M., Toledo-Crow, R. & Miesenbock, G. Video-rate nonlinear microscopy of neuronal membrane dynamics with genetically encoded probes. J. Neurophysiol. 92, 609–621 (2004)
Kremer, Y. et al. A spatio-temporally compensated acousto-optic scanner for two-photon microscopy providing large field of view. Opt. Express 16, 10066–10076 (2008)
Gerig, J. S. & Montague, H. A simple optical filter for chirp radar. Proc. IEEE 52, 1753 (1964)
Kerlin, A. M., Andermann, M. L., Berezovskii, V. K. & Reid, R. C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010)
Sohya, K., Kameyama, K., Yanagawa, Y., Obata, K. & Tsumoto, T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J. Neurosci. 27, 2145–2149 (2007)
Peters, A. & Jones, E. G. in Cerebral Cortex: Cellular Components of the Cerebral Cortex Vol. 1 (eds Peters, A. & Jones, E. G.) 107–121 (Plenum, 1984)
Kawaguchi, Y., Karube, F. & Kubota, Y. Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cereb. Cortex 16, 696–711 (2006)
Holtmaat, A. J. et al. Transient and persistent dendritic spines in the neocortex in vivo . Neuron 45, 279–291 (2005)
Acknowledgements
We thank J. Lou for technical assistance, D. Bayer, F. Bayer and W. Zeitz for building the scanning device, A. Fohr for software support and Y. Kovalchuk and H. Adelsberger for help during the initial experiments. This work was supported by the Schiedel Foundation, the German-Israeli Foundation (GIF grant 1002/2008 to I.N. and A.K.), the Deutsche Forschungsgemeinschaft (IRTG 1373) and the Bundesministerium für Bildung und Forschung (BMBF) in the frame of ERA-NET NEURON. A.K. is a Carl-von-Linde senior fellow of the Institute for Advanced Study of the Technische Universität München.
Author information
Authors and Affiliations
Contributions
X.C., U.L., I.N., N.L.R. and A.K. carried out the experiments. U.L. and A.K. designed and constructed the imaging device. X.C., U.L., N.L.R., I.N. and A.K. performed the analysis. A.K. designed the study and wrote the manuscript with the help of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-11 with legends, Supplementary Table 1, the legend for Supplementary Movie 1 and additional references. (PDF 1020 kb)
Supplementary Movie 1
The movie shows three consecutive trials of spine calcium responses (S1-S4) to pure tone stimulation (0 dB attenuation; 100 ms duration) – see Supplementary Information file for full legend. (MOV 14577 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Leischner, U., Rochefort, N. et al. Functional mapping of single spines in cortical neurons in vivo. Nature 475, 501–505 (2011). https://doi.org/10.1038/nature10193
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10193
This article is cited by
-
Immediate reuse of patch-clamp pipettes after ultrasonic cleaning
Scientific Reports (2024)
-
Transport and localization on dendrite-inspired flat band linear photonic lattices
Scientific Reports (2023)
-
Cortical recurrence supports resilience to sensory variance in the primary visual cortex
Communications Biology (2023)
-
Generalized extinction of fear memory depends on co-allocation of synaptic plasticity in dendrites
Nature Communications (2023)
-
A silent two-photon imaging system for studying in vivo auditory neuronal functions
Light: Science & Applications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.