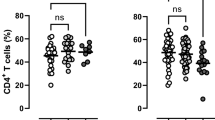
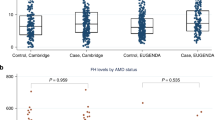
Abstract
Oxidative stress and enhanced lipid peroxidation are linked to many chronic inflammatory diseases, including age-related macular degeneration (AMD). AMD is the leading cause of blindness in Western societies, but its aetiology remains largely unknown. Malondialdehyde (MDA) is a common lipid peroxidation product that accumulates in many pathophysiological processes, including AMD. Here we identify complement factor H (CFH) as a major MDA-binding protein that can block both the uptake of MDA-modified proteins by macrophages and MDA-induced proinflammatory effects in vivo in mice. The CFH polymorphism H402, which is strongly associated with AMD, markedly reduces the ability of CFH to bind MDA, indicating a causal link to disease aetiology. Our findings provide important mechanistic insights into innate immune responses to oxidative stress, which may be exploited in the prevention of and therapy for AMD and other chronic inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chou, M. Y. et al. Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 263, 479–488 (2008)
Esterbauer, H., Schaur, R. J. & Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 (1991)
Chang, M. K. et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl Acad. Sci. USA 96, 6353–6358 (1999)
Miller, Y. I. et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 (2011)
Hollyfield, J. G. et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Med. 14, 194–198 (2008)
Suzuki, M. et al. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol. Vis. 13, 772–778 (2007)
Jager, R. D., Mieler, W. F. & Miller, J. W. Age-related macular degeneration. N. Engl. J. Med. 358, 2606–2617 (2008)
Schutt, F., Bergmann, M., Holz, F. G. & Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 44, 3663–3668 (2003)
Thiele, G. M. et al. Malondialdehyde-acetaldehyde (MAA) modified proteins induce pro-inflammatory and pro-fibrotic responses by liver endothelial cells. Comp. Hepatol. 3 (suppl. 1). S25 (2004)
Shanmugam, N. et al. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes 57, 879–888 (2008)
Shechter, I. et al. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J. Lipid Res. 22, 63–71 (1981)
Chou, M. Y. et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 119, 1335–1349 (2009)
Xu, D. et al. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem. Res. Toxicol. 10, 978–986 (1997)
Zipfel, P. F. & Skerka, C. Complement regulators and inhibitory proteins. Nature Rev. Immunol. 9, 729–740 (2009)
Józsi, M. & Zipfel, P. F. Factor H family proteins and human diseases. Trends Immunol. 29, 380–387 (2008)
Haines, J. L. et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421 (2005)
Klein, R. J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389 (2005)
Edwards, A. O. et al. Complement factor H polymorphism and age-related macular degeneration. Science 308, 421–424 (2005)
Hageman, G. S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl Acad. Sci. USA 102, 7227–7232 (2005)
Hughes, A. E. et al. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nature Genet. 38, 1173–1177 (2006)
Takizawa, F., Tsuji, S. & Nagasawa, S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 397, 269–272 (1996)
Amarilyo, G. et al. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-κB-dependent blockade. Eur. J. Immunol. 40, 699–709 (2010)
Higgins, G. T., Wang, J. H., Dockery, P., Cleary, P. E. & Redmond, H. P. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest. Ophthalmol. Vis. Sci. 44, 1775–1782 (2003)
de Jong, P. T. Age-related macular degeneration. N. Engl. J. Med. 355, 1474–1485 (2006)
Binder, C. J. et al. Innate and acquired immunity in atherogenesis. Nature Med. 8, 1218–1226 (2002)
Flierman, R. & Daha, M. R. The clearance of apoptotic cells by complement. Immunobiology 212, 363–370 (2007)
Trouw, L. A. et al. C4b-binding protein and factor H compensate for the loss of membrane-bound complement inhibitors to protect apoptotic cells against excessive complement attack. J. Biol. Chem. 282, 28540–28548 (2007)
Meri, S. & Pangburn, M. K. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc. Natl Acad. Sci. USA 87, 3982–3986 (1990)
Leffler, J. et al. Annexin-II, DNA, and histones serve as factor H ligands on the surface of apoptotic cells. J. Biol. Chem. 285, 3766–3776 (2010)
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nature Rev. Immunol. 2, 965–975 (2002)
Chang, M. K. et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 200, 1359–1370 (2004)
Mihlan, M., Stippa, S., Jozsi, M. & Zipfel, P. F. Monomeric CRP contributes to complement control in fluid phase and on cellular surfaces and increases phagocytosis by recruiting factor H. Cell Death Differ. 16, 1630–1640 (2009)
Thakkinstian, A. et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum. Mol. Genet. 15, 2784–2790 (2006)
Donoso, L. A., Vrabec, T. & Kuivaniemi, H. The role of complement factor H in age-related macular degeneration: a review. Surv. Ophthalmol. 55, 227–246 (2010)
Clark, S. J. et al. His-384 allotypic variant of factor H associated with age-related macular degeneration has different heparin binding properties from the non-disease-associated form. J. Biol. Chem. 281, 24713–24720 (2006)
Green, W. R. & Enger, C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 100, 1519–1535 (1993)
Sun, M. et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 281, 4222–4230 (2006)
Kaemmerer, E., Schutt, F., Krohne, T. U., Holz, F. G. & Kopitz, J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest. Ophthalmol. Vis. Sci. 48, 1342–1347 (2007)
Goverdhan, S. V. et al. Interleukin-8 promoter polymorphism −251A/T is a risk factor for age-related macular degeneration. Br. J. Ophthalmol. 92, 537–540 (2008)
Sofat, R. et al. Genetic variation in complement factor H and risk of coronary heart disease: eight new studies and a meta-analysis of around 48,000 individuals. Atherosclerosis 213, 184–190 (2010)
Scholl, H. P. et al. Systemic complement activation in age-related macular degeneration. PLoS ONE 3, e2593 (2008)
Binder, C. J. et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature Med. 9, 736–743 (2003)
Acknowledgements
We are indebted to M. Ozsvar-Kozma for technical assistance, S. Hälbich for performing the surface plasmon resonance analysis, A. Hartmann for purification of CFH variants from patient plasma, C. Mannhalter for help with genotyping, and E. N. Montano. This work was supported by the Austrian Academy of Sciences, a BRIDGE grant from the Austrian Research Promotion Agency, the SFB Lipotox F30 of the Austrian Science Fund (C.J.B.); NIH grants HL088093 (C.J.B., S.T., J.L.W.), RO1 HL086599 (K.H., J.L.W.), EY14005, EY019044 (J.T.H.); the Edward N. & Della L. Thome Memorial Foundation Awards Program in AMD Research, Research to Prevent Blindness (Wilmer Eye institute) (J.T.H.); the Deutsche Forschungsgemeinschaft (P.F.Z., C.S.); the ProRetina Foundation (N.L., P.F.Z., C.S.); the Fondation Leducq (C.J.B., S.T., J.L.W.); the Wynn-Gund Translational Research Acceleration Program Enhanced Research and Clinical Training Award, National Neurovision Research Institute – Foundation Fighting Blindness, Macular Degeneration Research Award, American Health Assistance Foundation (H.P.N.S.); European Commission and the Seventh European Community Framework Program, Marie Curie Intra-European Fellowship (P.C.I.). K.H. was supported by the Scientist Development Grant 0630228N of the American Heart Association. J.T.H. is the Robert Bond Welch Professor. We thank all patients for participation.
Author information
Authors and Affiliations
Contributions
D.W. and C.J.B. conceived the project and designed and analysed the experiments with contributions from J.L.W., P.F.Z., J.T.H., G.S.-F. and C.S.; D.W. performed most of the experiments, with contributions from K.H., N.L., K.L.B., M.C., S.T. and H.B.; H.P.N.S. and P.C.I. obtained and provided AMD plasma samples; D.W. and C.J.B. wrote the manuscript with contributions from J.L.W., P.F.Z. and J.T.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-15 with legends, Supplementary Methods, which includes 3 tables and additional references and Supplementary Table 1. (PDF 1367 kb)
Rights and permissions
About this article
Cite this article
Weismann, D., Hartvigsen, K., Lauer, N. et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81 (2011). https://doi.org/10.1038/nature10449
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10449
This article is cited by
-
A guide to complement biology, pathology and therapeutic opportunity
Nature Reviews Immunology (2024)
-
Antibodies in action: the role of humoral immunity in the fight against atherosclerosis
Immunity & Ageing (2022)
-
Unravelling the therapeutic potential of IL-33 for atrophic AMD
Eye (2022)
-
Isotypes of autoantibodies against novel differential 4-hydroxy-2-nonenal-modified peptide adducts in serum is associated with rheumatoid arthritis in Taiwanese women
BMC Medical Informatics and Decision Making (2021)
-
Multi-omic analysis unveils biological pathways in peripheral immune system associated to minimal hepatic encephalopathy appearance in cirrhotic patients
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.