Abstract
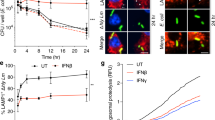
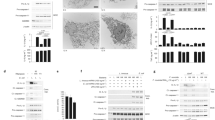
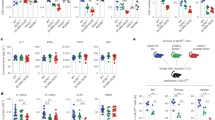
Members of the intracellular nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) family contribute to immune responses through activation of nuclear factor-κB (NF-κB), type I interferon and inflammasome signalling1. Mice lacking the NLR family member NLRP6 were recently shown to be susceptible to colitis and colorectal tumorigenesis2,3,4, but the role of NLRP6 in microbial infections and the nature of the inflammatory signalling pathways regulated by NLRP6 remain unclear. Here we show that Nlrp6-deficient mice are highly resistant to infection with the bacterial pathogens Listeria monocytogenes, Salmonella typhimurium and Escherichia coli. Infected Nlrp6-deficient mice had increased numbers of monocytes and neutrophils in circulation, and NLRP6 signalling in both haematopoietic and radioresistant cells contributed to increased susceptibility. Nlrp6 deficiency enhanced activation of mitogen-activated protein kinase (MAPK) and the canonical NF-κB pathway after Toll-like receptor ligation, but not cytosolic NOD1/2 ligation, in vitro. Consequently, infected Nlrp6-deficient cells produced increased levels of NF-κB- and MAPK-dependent cytokines and chemokines. Thus, our results reveal NLRP6 as a negative regulator of inflammatory signalling, and demonstrate a role for this NLR in impeding clearance of both Gram-positive and -negative bacterial pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kanneganti, T. D., Lamkanfi, M. & Nunez, G. Intracellular NOD-like receptors in host defense and disease. Immunity 27, 549–559 (2007)
Chen, G. Y., Liu, M., Wang, F., Bertin, J. & Nunez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 (2011)
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011)
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl Acad. Sci. USA 108, 9601–9606 (2011)
Centers for Disease Control and Prevention. CDC Estimates of Foodbornes Illness in the United States, 2011 Estimates. http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html
Scallan, E. et al. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17, 7–15 (2011)
Anand, P. K. et al. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via ERK activation. J. Biol. Chem. 286, 42981–42991 (2011)
Corr, S. C. & O'Neill, L. A. Listeria monocytogenes infection in the face of innate immunity. Cell. Microbiol. 11, 703–709 (2009)
Hybiske, K. & Stephens, R. S. Exit strategies of intracellular pathogens. Nature Rev. Microbiol. 6, 99–110 (2008)
Creagh, E. M. & O'Neill, L. A. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27, 352–357 (2006)
Kanneganti, T. D. Central roles of NLRs and inflammasomes in viral infection. Nature Rev. Immunol. 10, 688–698 (2010)
Pétrilli, V., Dostert, C., Muruve, D. A. & Tschopp, J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 (2007)
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012)
Grenier, J. M. et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-κB and caspase-1. FEBS Lett. 530, 73–78 (2002)
Stevenson, M. M., Kongshavn, P. A. & Skamene, E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J. Immunol. 127, 402–407 (1981)
Lara-Tejero, M. et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203, 1407–1412 (2006)
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunol. 7, 311–317 (2006)
Meixenberger, K. et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1β, depending on listeriolysin O and NLRP3. J. Immunol. 184, 922–930 (2010)
Waterfield, M. R., Zhang, M., Norman, L. P. & Sun, S. C. NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol. Cell 11, 685–694 (2003)
van Ooij, C. Immunology: NLRP6 keeps the bad bacteria at bay. Nature Rev. Microbiol. 9, 481 (2011)
Girardin, S. E. et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003)
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005)
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunol. 7, 576–582 (2006)
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010)
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010)
Portnoy, D. A., Jacks, P. S. & Hinrichs, D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167, 1459–1471 (1988)
Acknowledgements
We thank A. Coyle, E. Grant and R. Flavell for the supply of mutant mice. M.L. is supported by grants from the European Union Framework Program 7 (Marie-Curie Grant 256432), European Research Council (grant 281600) and the Fund for Scientific Research Flanders (G030212N, 1.2.201.10.N.00 and 1.5.122.11.N.00). This work was supported by National Institute of Health Grants (AR056296 and AI101935), and the American Lebanese Syrian Associated Charities (ALSAC) to T.-D.K.
Author information
Authors and Affiliations
Contributions
T.-D.K., M.L. and P.K.A. designed the research; P.K.A., R.K.S.M. and J.R.L. performed the experiments; P.V. performed and analysed the histopathology data; J.B. provided essential reagents; T.-D.K., M.L., P.K.A., R.K.S.M., J.R.L., P.V. and J.B. analysed the data; P.K.A., M.L. and T.-D.K. wrote the paper; and T.-D.K. conceived the study, designed the experiments and provided overall direction. M.L. and T.-D.K. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-16. (PDF 1397 kb)
Rights and permissions
About this article
Cite this article
Anand, P., Malireddi, R., Lukens, J. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012). https://doi.org/10.1038/nature11250
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11250
This article is cited by
-
Microbiota-dependent regulation of costimulatory and coinhibitory pathways via innate immune sensors and implications for immunotherapy
Experimental & Molecular Medicine (2023)
-
The NLR gene family: from discovery to present day
Nature Reviews Immunology (2023)
-
NLRP6 potentiates PI3K/AKT signalling by promoting autophagic degradation of p85α to drive tumorigenesis
Nature Communications (2023)
-
Amyloid-β mediates intestinal dysfunction and enteric neurons loss in Alzheimer's disease transgenic mouse
Cellular and Molecular Life Sciences (2023)
-
Association of NLRPs with pathogenesis of dry age-related macular degeneration
International Ophthalmology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.