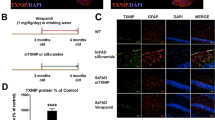
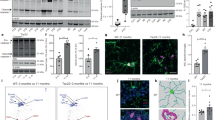
Abstract
Alzheimer’s disease is the world’s most common dementing illness. Deposition of amyloid-β peptide drives cerebral neuroinflammation by activating microglia1,2. Indeed, amyloid-β activation of the NLRP3 inflammasome in microglia is fundamental for interleukin-1β maturation and subsequent inflammatory events3. However, it remains unknown whether NLRP3 activation contributes to Alzheimer’s disease in vivo. Here we demonstrate strongly enhanced active caspase-1 expression in human mild cognitive impairment and brains with Alzheimer’s disease, suggesting a role for the inflammasome in this neurodegenerative disease. Nlrp3−/− or Casp1−/− mice carrying mutations associated with familial Alzheimer’s disease were largely protected from loss of spatial memory and other sequelae associated with Alzheimer’s disease, and demonstrated reduced brain caspase-1 and interleukin-1β activation as well as enhanced amyloid-β clearance. Furthermore, NLRP3 inflammasome deficiency skewed microglial cells to an M2 phenotype and resulted in the decreased deposition of amyloid-β in the APP/PS1 model of Alzheimer’s disease. These results show an important role for the NLRP3/caspase-1 axis in the pathogenesis of Alzheimer’s disease, and suggest that NLRP3 inflammasome inhibition represents a new therapeutic intervention for the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
30 January 2013
Minor corrections were made to Fig. 2d and the legend to Fig. 3.
References
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nature Neurosci. 14, 1227–1235 (2011)
Lucin, K. M. & Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64, 110–122 (2009)
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nature Immunol. 9, 857–865 (2008)
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009)
Jankowsky, J. L. et al. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol. Eng. 17, 157–165 (2001)
Bliss, T. V. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993)
Ho, V. M., Lee, J.-A. & Martin, K. C. The cell biology of synaptic plasticity. Science 334, 623–628 (2011)
Walker, J. M. et al. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease. Behav. Brain Res. 222, 169–175 (2011)
Heneka, M. T. & O’Banion, M. K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 184, 69–91 (2007)
Lee, C. Y. D. & Landreth, G. E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 (2010)
Nalbantoglu, J. et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature 387, 500–505 (1997)
Chapman, P. F. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neurosci. 2, 271–276 (1999)
Murray, C. A. & Lynch, M. A. Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age- and stress-induced impairments in long-term potentiation. J. Neurosci. 18, 2974–2981 (1998)
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nature Med. 13, 432–438 (2007)
Bamberger, M. E., Harris, M. E., McDonald, D. R., Husemann, J. & Landreth, G. E. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J. Neurosci. 23, 2665–2674 (2003)
Hickman, S. E., Allison, E. K. & Khoury, J. E. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 28, 8354–8360 (2008)
Heneka, M. T. et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl Acad. Sci. USA 107, 6058–6063 (2010)
Shaftel, S. S. et al. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 117, 1595–1604 (2007)
Shaftel, S. S. et al. Chronic interleukin-1β expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 27, 9301–9309 (2007)
Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 31, 11159–11171 (2011)
Malito, E., Hulse, R. E. & Tang, W.-J. Amyloid β-degrading cryptidases: insulin degrading enzyme, neprilysin, and presequence peptidase. Cell. Mol. Life Sci. 65, 2574–2585 (2008)
Leissring, M. A. et al. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 (2003)
Mawuenyega, K. G. et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330, 1774 (2010)
Raes, G. et al. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev. Immunol. 9, 151–159 (2002)
Kummer, M. P. et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron 71, 833–844 (2011)
Wang, Q., Rowan, M. J. & Anwyl, R. β-Amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J. Neurosci. 24, 6049–6056 (2004)
Kanneganti, T.-D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006)
Li, P. et al. Mice deficient in IL-1 β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995)
Bevins, R. A. & Besheer, J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature Protocols 1, 1306–1311 (2006)
Jäger, S. et al. alpha-secretase mediated conversion of the amyloid precursor protein derived membrane stub C99 to C83 limits Aβ generation. J. Neurochem. 111, 1369–1382 (2009)
Acknowledgements
This work was funded by the Dana Foundation (E.L.), the National Institutes of Health (E.L., D.T.G.) and the Deutsche Forschungsgemeinschaft (E.L., M.T.H.). We thank G. Nuñez and V. M. Dixit for providing anti-caspase-1 Abs. We thank B. De Strooper and L. Serneels for the BACE1 knockout mice and discussion. We also thank H. Jacobsen for the BACE1 transgenic mice.
Author information
Authors and Affiliations
Contributions
M.T.H, M.P.K, A.S., A.D., S.S., A.V.-S., A.G., D.A., A.R., T.T. and E.L. performed experiments and analysed data, E.G. provided human samples and analysed data, A.H. was involved in study design and analysed data, E.L., M.T.H., M.K. and D.T.G. designed the study and wrote the paper. All authors discussed results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-31. (PDF 3593 kb)
Rights and permissions
About this article
Cite this article
Heneka, M., Kummer, M., Stutz, A. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013). https://doi.org/10.1038/nature11729
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11729
This article is cited by
-
Hotspots and trends of microglia in Alzheimer's disease: a bibliometric analysis during 2000–2022
European Journal of Medical Research (2024)
-
The role of NLRP3 inflammasome in aging and age-related diseases
Immunity & Ageing (2024)
-
Inhibition of MMP8 effectively alleviates manic-like behavior and reduces neuroinflammation by modulating astrocytic CEBPD
Journal of Neuroinflammation (2024)
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.