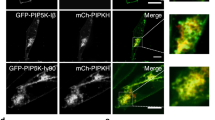
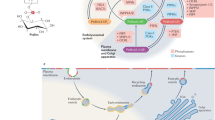
Abstract
Phosphoinositides serve crucial roles in cell physiology, ranging from cell signalling to membrane traffic1,2. Among the seven eukaryotic phosphoinositides the best studied species is phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), which is concentrated at the plasma membrane where, among other functions, it is required for the nucleation of endocytic clathrin-coated pits3,4,5,6. No phosphatidylinositol other than PI(4,5)P2 has been implicated in clathrin-mediated endocytosis, whereas the subsequent endosomal stages of the endocytic pathway are dominated by phosphatidylinositol-3-phosphates(PI(3)P)7. How phosphatidylinositol conversion from PI(4,5)P2-positive endocytic intermediates to PI(3)P-containing endosomes is achieved is unclear. Here we show that formation of phosphatidylinositol-3,4-bisphosphate (PI(3,4)P2) by class II phosphatidylinositol-3-kinase C2α (PI(3)K C2α) spatiotemporally controls clathrin-mediated endocytosis. Depletion of PI(3,4)P2 or PI(3)K C2α impairs the maturation of late-stage clathrin-coated pits before fission. Timed formation of PI(3,4)P2 by PI(3)K C2α is required for selective enrichment of the BAR domain protein SNX9 at late-stage endocytic intermediates. These findings provide a mechanistic framework for the role of PI(3,4)P2 in endocytosis and unravel a novel discrete function of PI(3,4)P2 in a central cell physiological process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Di Paolo, G. & De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 (2006)
Wymann, M. P. & Schneiter, R. Lipid signalling in disease. Nature Rev. Mol. Cell Biol. 9, 162–176 (2008)
Krauss, M., Kukhtina, V., Pechstein, A. & Haucke, V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2μ-cargo complexes. Proc. Natl Acad. Sci. USA 103, 11934–11939 (2006)
Loerke, D. et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 7, e57 (2009)
McMahon, H. T. & Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Rev. Mol. Cell Biol. 12, 517–533 (2011)
Zoncu, R. et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl Acad. Sci. USA 104, 3793–3798 (2007)
Gruenberg, J. Lipids in endocytic membrane transport and sorting. Curr. Opin. Cell Biol. 15, 382–388 (2003)
Antonescu, C. N., Aguet, F., o, Danuser, G. & Schmid, S. L. Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol. Biol. Cell 22, 2588–2600 (2011)
Chang-Ileto, B. et al. Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev. Cell 20, 206–218 (2011)
Shin, H.-W. et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 170, 607–618 (2005)
Bae, Y. H. et al. Profilin1 regulates PI(3,4)P2 and lamellipodin accumulation at the leading edge thus influencing motility of MDA-MB-231 cells. Proc. Natl Acad. Sci. USA 107, 21547–21552 (2010)
Dowler, S. et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19–31 (2000)
Gewinner, C. et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 16, 115–125 (2009)
Fili, N., Calleja, V., Woscholski, R., Parker, P. J. & Larijani, B. Compartmental signal modulation: endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc. Natl Acad. Sci. USA 103, 15473–15478 (2006)
Ferguson, S. M. et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell 17, 811–822 (2009)
Rameh, L. E. & Cantley, L. C. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274, 8347–8350 (1999)
Gaidarov, I., Smith, M. E., Domin, J. & Keen, J. H. The class II phosphoinositide 3-kinase C2α is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell 7, 443–449 (2001)
Stahelin, R. V. et al. Structural and membrane binding analysis of the Phox homology domain of phosphoinositide 3-kinase-C2α. J. Biol. Chem. 281, 39396–39406 (2006)
Domin, J. et al. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 326, 139–147 (1997)
Borner, G. H. H. et al. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J. Cell Biol. 197, 141–160 (2012)
Falasca, M. et al. The role of phosphoinositide 3-kinase C2α in insulin signaling. J. Biol. Chem. 282, 28226–28236 (2007)
Leibiger, B. et al. Insulin-feedback via PI3K–C2α activated PKBα/Akt1 is required for glucose-stimulated insulin secretion. FASEB J. 24, 1824–1837 (2010)
Pirola, L. et al. Activation loop sequences confer substrate specificity to phosphoinositide 3-kinase α (PI3Kα). Functions of lipid kinase-deficient PI3Kα in signaling. J. Biol. Chem. 276, 21544–21554 (2001)
Subramanian, D. et al. Activation of membrane-permeant caged PtdIns(3)P induces endosomal fusion in cells. Nature Chem. Biol. 6, 324–326 (2010)
Park, J. et al. SNX18 shares a redundant role with SNX9 and modulates endocytic trafficking at the plasma membrane. J. Cell Sci. 123, 1742–1750 (2010)
Yarar, D., Surka, M. C., Leonard, M. C. & Schmid, S. L. SNX9 activities are regulated by multiple phosphoinositides through both PX and BAR domains. Traffic 9, 133–146 (2008)
Rink, J., Ghigo, E., Kalaidzidis, Y. & Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 (2005)
Malecz, N. et al. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr. Biol. 10, 1383–1386 (2000)
Varnai, P., Thyagarajan, B., Rohacs, T. & Balla, T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175, 377–382 (2006)
Gaidarov, I., Zhao, Y. & Keen, J. H. Individual phosphoinositide 3-kinase C2α domain activities independently regulate clathrin function. J. Biol. Chem. 280, 40766–40772 (2005)
Pylypenko, O., Lundmark, R., Rasmuson, E., Carlsson, S. R. & Rak, A. The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J. 26, 4788–4800 (2007)
Maritzen, T. et al. Gadkin negatively regulates cell spreading and motility via sequestration of the actin-nucleating ARP2/3 complex. Proc. Natl Acad. Sci. USA 109, 10382–10387 (2012)
Laketa, V. et al. Membrane-permeant phosphoinositide derivatives as modulators of growth factor signaling and neurite outgrowth. Chem. Biol. 16, 1190–1196 (2009)
von Kleist, L. et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 (2011)
Campbell, C., Squicciarini, J., Shia, M., Pilch, P. F. & Fine, R. E. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry 23, 4420–4426 (1984)
Wieffer, M., Haucke, V. & Krauss, M. Regulation of phosphoinositide-metabolizing enzymes by clathrin coat proteins. Methods Cell Biol. 108, 209–225 (2012)
Acknowledgements
We thank E. Ungewickell, P. Di Fiore, P. De Camilli, H. McMahon, E. Wancker, T. Südhof and S. Carlsson for antibodies, L. Cantley, T. Takenawa, M. Wymann, T. Ross, O. Daumke and W. Yang for plasmids, and O. Daumke, B. Eickolt and F. Wieland for critical comments. Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 740/C8; SFB 740/D7; SFB 958/A04; SFB 958/A07; SFB 958/Z02).
Author information
Authors and Affiliations
Contributions
Y.P., M.E.-G., D.P., M.K. performed experiments; R.M., S.Z., C.S. provided reagents; A.L. and J.S. aided with microscopy; Y.P., M.E.-G., J.S., F.N. and V.H. designed research; F.G. and E.H. contributed reagents; J.S., A.U. and. F.N. conducted simulations. Y.P., F.N. and V.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 and Supplementary Table 1. (PDF 10942 kb)
Depletion of PI(3,4)P2 attenuates CCP dynamics
The peripheral regions of two neighbouring eGFP-clathrin light chain expressing cells imaged by TIRF microscopy shown for 3 min. The cell on the right suffers from PI(3,4)P2 depletion due to co-expression of mCherry-INPP4B-CAAX . For clarity, a dotted line has been drawn along the border between the two cells. Note the strikingly attenuated CCPs dynamics in the PI(3,4)P2-depleted cell. (MOV 7431 kb)
Attenuated CCP dynamics upon depletion of PI3K C2α
Videos 2 and 3 show representative areas from eGFP-clathrin light chain expressing cells treated with scrambled or PI3K C2α siRNAs, respectively, imaged by TIRF microscopy for 3 min. CCPs in control cells (video 2; scrambled siRNA) display a dynamic succession of appearance, growth, and disappearance (internalization). By contrast, CCPs in PI3K C2α-depleted cells (video 3; PI3K C2α-siRNA) are long-lived and stable over time, indicative of defective CCP maturation. (MOV 4251 kb)
Attenuated CCP dynamics upon depletion of PI3K C2α
Videos 2 and 3 show representative areas from eGFP-clathrin light chain expressing cells treated with scrambled or PI3K C2α siRNAs, respectively, imaged by TIRF microscopy for 3 min. CCPs in control cells (video 2; scrambled siRNA) display a dynamic succession of appearance, growth, and disappearance (internalization). By contrast, CCPs in PI3K C2α-depleted cells (video 3; PI3K C2α-siRNA) are long-lived and stable over time, indicative of defective CCP maturation. (MOV 3910 kb)
Rights and permissions
About this article
Cite this article
Posor, Y., Eichhorn-Gruenig, M., Puchkov, D. et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499, 233–237 (2013). https://doi.org/10.1038/nature12360
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12360
This article is cited by
-
Effect of hormone-induced plasma membrane phosphatidylinositol 4,5-bisphosphate depletion on receptor endocytosis suggests the importance of local regulation in phosphoinositide signaling
Scientific Reports (2024)
-
Generation of nanoscopic membrane curvature for membrane trafficking
Nature Reviews Molecular Cell Biology (2023)
-
Beyond PI3Ks: targeting phosphoinositide kinases in disease
Nature Reviews Drug Discovery (2023)
-
Development of selective inhibitors of phosphatidylinositol 3-kinase C2α
Nature Chemical Biology (2023)
-
Intercellular transfer of activated STING triggered by RAB22A-mediated non-canonical autophagy promotes antitumor immunity
Cell Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.