Abstract
arising from N. C. Derecki et al. Nature 484, 105–109 (2012); doi:10.1038/nature10907
Rett syndrome is a severe neurodevelopmental disorder caused by mutations in the X chromosomal gene MECP2 (ref. 1), and its treatment so far is symptomatic. Mecp2 disruption in mice phenocopies major features of the syndrome2 that can be reversed after Mecp2 re-expression3. Recently, Derecki et al.4 reported that transplantation of wild-type bone marrow into lethally irradiated Mecp2-null (Mecp2tm1.1Jae/y) mice prevented neurological decline and early death by restoring microglial phagocytic activity against apoptotic targets4, and clinical trials of bone marrow transplantation (BMT) for patients with Rett syndrome have thus been initiated5. We aimed to replicate and extend the BMT experiments in three different Rett syndrome mouse models, but found that despite robust microglial engraftment, BMT from wild-type donors did not prevent early death or ameliorate neurological deficits. Furthermore, early and specific Mecp2 genetic expression in microglia did not rescue Mecp2-deficient mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Neul, J. L. The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin. Neurosci. 14, 253–262 (2012).
Katz, D. M. et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Model. Mech. 5, 733–745 (2012).
Guy, J., Gan, J., Selfridge, J., Cobb, S. & Bird, A. Reversal of neurological defects in a mouse model of Rett syndrome, Science 315, 1143–1147 (2007).
Derecki, N. C. et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105–109 (2012).
MT2013-31:. Allogeneic hematopoietic cell transplantation for inherited metabolic disorders, sever osteoporosis and males with Rett syndrome following conditioning with busulfan (therapeutic drug monitoring), fludarabine +/− ATG; Masconic Cancer Center, University of Minnesota. ClinicalTrials.gov identifier: NCT02171104 (2014).
Landis, S. C. et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191 (2012).
Yang, Y. et al. Perivascular, but not parenchymal, cerebral engraftment of donor cells after non-myeloablative bone marrow transplantation. Exp. Mol. Pathol. 95, 7–17 (2013).
Brendel, C. et al. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J. Mol. Med. (Berl.) 89, 389–398 (2011).
Pitcher, M. R. et al. Insulinotropic treatments exacerbate metabolic syndrome in mice lacking MeCP2 function. Hum. Mol. Genet. 22, 2626–2633 (2013).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nature Neurosci. 16, 1618–1626 (2013).
Sasmono, R. T. et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101, 1155–1163 (2003).
Chen, R. T., Akbarian, S., Tudor, M. & Jaenisch, R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genet. 27, 327–331 (2001).
Author information
Authors and Affiliations
Contributions
All experiments were conceived of and supervised by P.H., J.L.N., A.B. and A.A.P., who are jointly responsible for the work presented and are corresponding authors. Designed experiments: A.A.P., A.B., S.S., V.L., J.W., P.X., R.S., D.J.B., J.L.N., T.-W.H., C.S.W., D.P., S.C., M.G., J.E.W., P.H., M.J.Y., J.L.J., S.D.R., H.M.R., J.G. Collected data: B.N., C.N., U.L., D.A.F., N.L.J., Y.Y., S.V., L.G., J.W., P.X., S.T., W.K., J.B., R.S., L.M., D.J.B., T.-W.H., C.S.W., D.P., S.C., J.E.W., M.J.Y., J.L.J., S.D.R., H.M.R. Analysed data: A.A.P., A.B., J.W., K.L., M.F., C.D.K., I.B., H.D.J.-C., P.X., R.S., D.J.B., J.L.N., T.-W.H., C.S.W., D.P., S.C., M.G., J.E.W., P.H., S.D.R., H.M.R., J.G. Wrote manuscript: A.A.P., A.B., J.W., D.J.B., H.D.J.-C., J.L.N., T.-W.H., P.H., J.E.W. Edited manuscript: A.A.P., A.B., M.S.B., C.D.K., I.B., H.D.J.-C., D.J.B., J.L.N., T.-W.H., C.S.W., D.P., S.C., M.G., P.H., J.G., H.M.R.
Corresponding authors
Ethics declarations
Competing interests
Competing Financial Interests Declared none.
Extended data figures and tables
Extended Data Figure 1 Engraftment with donor cells after bone marrow transplantation, and lack of evidence of neuropathology in Mecp2-null animals.
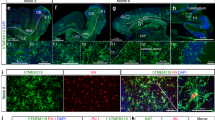
a, Multilineage peripheral blood engraftment with donor cells in Mecp2tm1.1Jae/y and wild-type mice. Wild-type and Mecp2tm1.1Jae/y animals received transplant from wild-type animals ubiquitously expressing GFP (Jackson Labs, C57BL/6-Tg(UBC-GFP)20Scha/J, stock 004353). Peripheral blood engraftment in indicated blood lineages was measure by flow cytometry (GFP) 4 and 8 weeks after transplant. b, PCR analysis of blood and tail tissue 4 weeks after transplant. Expression of only the appropriate mutant or wild-type variant of Mecp2 from the donor in blood in all four groups is shown, with retention of the original genotype in tail tissue as expected. Specifically, Mecp2+/y to Mecp2+/y mice show only the wild-type allele at 190 base pairs (bp), whereas Mecp2tm1.1Jae/y to Mecp2tm1.1Jae/y mice show only the mutant allele at 250 bp, as previously described in the original report of generation of these mice12 . Mecp2tm1.1Jae/y to Mecp2+/y mice, however, show only the mutant allele in blood tissue and retention of host wild-type allele in tail tissue. Accordingly, Mecp2+/y to Mecp2tm1.1Jae/y mice show only the wild-type allele in blood tissue, with retention of the host mutant allele in tail tissue. Tail tissue in these latter two groups shows some of the allele from the donor as well, presumably owing to blood contained within the tail clips used for analysis. Notably, the Mecp2 allele expressed in blood is always restricted to the donor genotype, indicating successful transplantation with complete replacement of the haematopoietic system in the host. Samples are labelled with a ‘T’ for tail and ‘B’ for blood, followed by the number of the animal, indicating that six different animals were analysed for each condition. CTD, C terminus domain α and β; HMGD1/2, high mobility group protein-like domain 1/2; MBD, methyl binding domain; NLS, nuclear localization signal; TRD, transcription repression domain. c, Robust and early microglial engraftment of donor cells after BMT in Mecp2tm1.1Jae/y and wild-type mice. Microglial engraftment was visualized using double immunofluorescence staining in sections quenched for autofluorescence by incubation in Sudan black solution. All sections were stained with an anti-Iba1 primary with CY-3 secondary and an anti-GFP primary with CY-5 secondary. All microglia are Iba1-positive, and thus successfully engrafted GFP-expressing donor-derived microglia were observed as GFP+/Iba1+, whereas native microglia were only Iba1+. Engraftment of microglia into wild-type and Mecp2LucHyg/y mice was determined by dividing the GFP+/Iba1+ cells by the number of total Iba1+ cells. Cell counts were performed in cerebellum, cortex and brainstem from mice. Percentage engraftment in wild-type and Mecp2tm1.1Jae/y mice yielded similar results to previously published engraftment results at 30 days after transplantation9. d, BMT was well-tolerated in animals. No difference in survival was observed in mutant animals that received Mecp2+/y marrow from their wild-type littermates (n = 13) and C57Bl/6J animals ubiquitously expressing GFP (n = 13). KO, knockout. e, Representative haematoxylin-and-eosin-stained sections of cerebellum, brainstem and hippocampus from age-matched wild-type and Mecp2tm1.1Jae/y mice killed at 7 weeks of age. Original magnification, ×400. Sections demonstrate comparable histological features between wild-type and Mecp2tm1.1Jae/y brains, and a lack of gliosis, cell loss, cellular debris, microglia or macrophages in Mecp2tm1.1Jae/y brains.
Extended Data Figure 2 Early transplantation of wild-type microglia into the brain does not rescue additional models of Mecp2-null mice: Mecp2LucHyg mice and C57Bl/6J Mecp2R168X mice.
a, Generation of Mecp2LucHyg mice. Luciferase/hygromycin (LucHyg) fusion gene vector correctly targeted to the Mecp2 locus in embryonic stem cells. Positions of the probes and enzyme restriction sites are indicated. The homology arms of the targeting vector are depicted in black, and its backbone in grey. b, Confirmation of genetic targeting for Mecp2LucHyg mice. Southern blotting of NdeI- or KpnI-digested DNA extracted from clone C4 cells, used for blastocyst injections, hybridized with either the hygromycin or external probe confirms correctly targeted event. c, Luciferase activity in clone C4 cells before (day 0) or after (day 5) subjecting cells to retinoic-acid-induced differentiation. After adsorption to eliminate feeder mouse embryonic fibroblasts, clone C4 embryonic stem cells were treated with retinoic acid (100 nM) in differentiation medium for 5 days, and luciferase activity was measured before and after retinoic acid treatment. Mean values are plotted relative to that of the wild-type cells (n = 3, error bars denote s.d.). Retinoic-acid -induced differentiation leads to an increase in luciferase activity consistent with an increase in Mecp2 expression level as measured in d. d, mRNA levels of Mecp2 increased and of embryonic stem-cell marker Nanog decreased in clone C4 cells subjected to retinoic-acid-induced differentiation. mRNA levels were measured before and after treatment by qPCR. Mean values plotted relative to day 0 for each mRNA (n = 3, error bars denote s.d.). e, Western blot analysis of MECP2 expression in brains of wild-type and Mecp2LucHyg male mice. MECP2 protein is not detected in MECP2 luciferase males. Ponceau S staining serves as a loading control. f, g, Robust peripheral blood and microglial engraftment of donor cells after BMT in Mecp2LucHyg/y mice. Wild-type and Mecp2LucHyg/y mice received wild-type bone marrow marked with GFP or CD41.1. Peripheral blood engraftment was measured by flow cytometry (GFP or CD45.1) in the indicated lineage 4–8 weeks after transplantation. For central nervous system engraftment, flow cytometry was performed on isolated mononuclear cells from the cortex, brainstem, cerebellum, hippocampus and striatum. Engraftment of BMT-derived cells was determined by dividing the CD11b+CD45+GFP+ cell population by total CD11b+CD45+ monocytes/microglia. h, Robust peripheral blood engraftment of donor cells 7 weeks after BMT in Mecp2R168X mice. Reconstitution of bone marrow from B6.SJL-Ptprca Pepcb/BoyJ mice into wild-type mice and C57Bl/6J Mecp2R168X mice showed robust engraftment in peripheral blood. Reconstitution of bone marrow was determined by FACS analysis of peripheral blood using anti-GR-1, anti-CD4 and anti-CD8 antibodies and CD45.1 for the donor cells (B6.SJL-Ptprca Pepcb/BoyJ mice, white bars) and CD45.2 for host cells (wild-type and C57Bl/6J Mecp2R168X mice, grey bars).
Extended Data Figure 3 Flow sorting and histological characterization of LysM-Cre or Vav1-Cre transgenic mice.
a, Stepwise process to characterize the amount of microglia (CD45 lo expressing) cells that also express tdTomato in a LysM-Cre-dependent fashion. b, High power images of cortex from LysM-CreTg/+; Mecp2LSL/Y animals. Scale bars, 50 μm. c, Merged high power images from cortex, pons and medulla from LysM-Cre; Mecp2LSL/Y animal. Circumflex (^) symbols identify large NeuN staining cells that express MECP2 (NeuN+/MECP2+); downward-facing triangles mark microglia not expressing MECP2 (Iba1+/MECP2−). Scale bars, 20 μm. d, Gating strategy for microglia sorting for Mecp2 expression quantification in Vav1-Cre; Mecp2LSL/Y and control animals is presented: (i) size/complexity (size/cytoplasmic granularity for cells but not debris); (ii) forward scatter pulse height/area (eliminates doublet cells); (iii) side scatter pulse height/width (eliminates doublet cells); (iv) SYTOX red staining; dead cells are SYTOX-red-positive and removed from the following analysis; (v) fms-GFP expression analysis enables the purification of microglia.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Acknowledgements. (PDF 446 kb)
Supplementary data
This file contains genome scanning data. (XLS 383 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Wang, J., Wegener, J., Huang, TW. et al. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521, E1–E4 (2015). https://doi.org/10.1038/nature14444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14444
This article is cited by
-
Inhibition of MMP8 effectively alleviates manic-like behavior and reduces neuroinflammation by modulating astrocytic CEBPD
Journal of Neuroinflammation (2024)
-
Strategies for Manipulating Microglia to Determine Their Role in the Healthy and Diseased Brain
Neurochemical Research (2023)
-
Evolving Models and Tools for Microglial Studies in the Central Nervous System
Neuroscience Bulletin (2021)
-
P2X7 receptor inhibition ameliorates dendritic spine pathology and social behavioral deficits in Rett syndrome mice
Nature Communications (2020)
-
Rett syndrome: insights into genetic, molecular and circuit mechanisms
Nature Reviews Neuroscience (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.