Abstract
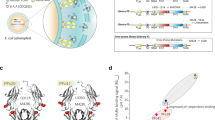
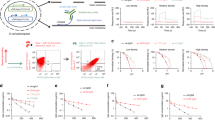
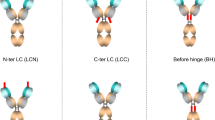
For many antibodies, each antigen-binding site binds to only one antigen molecule during the antibody's lifetime in plasma. To increase the number of cycles of antigen binding and lysosomal degradation, we engineered tocilizumab (Actemra)1, an antibody against the IL-6 receptor (IL-6R), to rapidly dissociate from IL-6R within the acidic environment of the endosome (pH 6.0) while maintaining its binding affinity to IL-6R in plasma (pH 7.4). Studies using normal mice and mice expressing human IL-6R2 suggested that this pH-dependent IL-6R dissociation within the acidic environment of the endosome resulted in lysosomal degradation of the previously bound IL-6R while releasing the free antibody back to the plasma to bind another IL-6R molecule. In cynomolgus monkeys, an antibody with pH-dependent antigen binding, but not an affinity-matured variant, significantly improved the pharmacokinetics and duration of C-reactive protein inhibition. Engineering pH dependency into the interactions of therapeutic antibodies with their targets may enable them to be delivered less frequently or at lower doses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mircic, M. & Kavanaugh, A. The clinical efficacy of tocilizumab in rheumatoid arthritis. Drugs Today (Barc) 45, 189–197 (2009).
Hirota, H., Yoshida, K., Kishimoto, T. & Taga, T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc. Natl. Acad. Sci. USA 92, 4862–4866 (1995).
Chan, A.C. & Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 10, 301–316 (2010).
Weiner, L.M., Surana, R. & Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 10, 317–327 (2010).
Ohsugi, Y. & Kishimoto, T. The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin. Biol. Ther. 8, 669–681 (2008).
Tabrizi, M.A., Tseng, C.M. & Roskos, L.K. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov. Today 11, 81–88 (2006).
Tabrizi, M., Bornstein, G.G. & Suria, H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 12, 33–43 (2010).
Ng, C.M., Stefanich, E., Anand, B.S., Fielder, P.J. & Vaickus, L. Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm. Res. 23, 95–103 (2006).
Kelley, S.K. et al. Preclinical pharmacokinetics, pharmacodynamics, and activity of a humanized anti-CD40 antibody (SGN-40) in rodents and non-human primates. Br. J. Pharmacol. 148, 1116–1123 (2006).
Bostrom, J., Lee, C.V., Haber, L. & Fuh, G. Improving antibody binding affinity and specificity for therapeutic development. Methods Mol. Biol. 525, 353–376 (2009).
Dall'Acqua, W.F., Kiener, P.A. & Wu, H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 281, 23514–23524 (2006).
Deng, R. et al. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab. Dispos. 38, 600–605 (2010).
Zalevsky, J. et al. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 28, 157–159 (2010).
Beck, A., Wurch, T., Bailly, C. & Corvaia, N. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 10, 345–352 (2010).
Rose-John, S., Scheller, J., Elson, G. & Jones, S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 80, 227–236 (2006).
Sato, K. et al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 15, 851–856 (1993).
Maxfield, F.R. & McGraw, T.E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5, 121–132 (2004).
Sarkar, C.A. et al. Rational cytokine design for increased lifetime and enhanced potency using pH-activated “histidine switching”. Nat. Biotechnol. 20, 908–913 (2002).
Maeda, K., Kato, Y. & Sugiyama, Y. pH-dependent receptor/ligand dissociation as a determining factor for intracellular sorting of ligands for epidermal growth factor receptors in rat hepatocytes. J. Control. Release 82, 71–82 (2002).
Burmeister, W.P., Huber, A.H. & Bjorkman, P.J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 372, 379–383 (1994).
Mihara, M. et al. Anti-interleukin 6 receptor antibody inhibits murine AA-amyloidosis. J. Rheumatol. 31, 1132–1138 (2004).
Finkelman, F.D. et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151, 1235–1244 (1993).
Shinkura, H. et al. In vivo blocking effects of a humanized antibody to human interleukin-6 receptor on interleukin-6 function in primates. Anticancer Res. 18, 1217–1221 (1998).
Genovese, M.C. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 58, 2968–2980 (2008).
Yasukawa, K. et al. Purification and characterization of soluble human IL-6 receptor expressed in CHO cells. J. Biochem. 108, 673–676 (1990).
Okazaki, M., Yamada, Y., Nishimoto, N., Yoshizaki, K. & Mihara, M. Characterization of anti-mouse interleukin-6 receptor antibody. Immunol. Lett. 84, 231–240 (2002).
Gerhartz, C. et al. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur. J. Biochem. 223, 265–274 (1994).
Acknowledgements
We thank T. Kishimoto at the Graduate School of Frontier Biosciences, Osaka University and T. Taga at the Kumamoto University Graduate School of Medical Sciences for kindly providing human IL-6R transgenic mice; colleagues in Chugai Research Institute for Medical Science, Inc., O. Ueda, T. Tachibe, M. Kakefuda and K. Jishage for breeding human IL-6R transgenic mice, T. Matsuura, M. Hiranuma, T. Koike, R. Takemoto, H. Azabu, T. Sakamoto, H. Sano and M. Kawaharada for carrying out in vivo experiments, and M. Fujii and A. Maeno for antibody vector construction, expression and purification; and colleagues in Chugai Pharmaceutical Co. Ltd., K. Kasutani, F. Mimoto and K. Esaki for carrying out in vitro experiments.
Author information
Authors and Affiliations
Contributions
T.I. led the overall pH-dependent binding antibody program, designed experiments, generated tocilizumab variants and wrote the manuscript. S.I. and A.M. generated tocilizumab variants. T.T., R.T., Y.H. and K. Haraya performed in vivo studies. S.S. led the anti-IL-6R antibody program. C.M. and A.H. performed affinity analysis of tocilizumab variants. T. Watanabe performed in vitro studies of tocilizumab variants. Y.D. and T. Wakabayashi performed purification of tocilizumab variants. S.K. and T.M. provided structural information for designing tocilizumab variants. Y.S., T.K., Y.N., Y.A., Y.K., and K. Hattori provided direction and guidance for the various functional areas.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of Chugai Pharmaceutical Co. Ltd.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–6 and Supplementary Figs. 1–4 (PDF 514 kb)
Rights and permissions
About this article
Cite this article
Igawa, T., Ishii, S., Tachibana, T. et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 28, 1203–1207 (2010). https://doi.org/10.1038/nbt.1691
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1691
This article is cited by
-
A pH-dependent anti-CD47 antibody that selectively targets solid tumors and improves therapeutic efficacy and safety
Journal of Hematology & Oncology (2023)
-
Modular cytokine receptor-targeting chimeras for targeted degradation of cell surface and extracellular proteins
Nature Biotechnology (2023)
-
Phase I study of MSB2311, a novel pH-dependent anti-PD-L1 monoclonal antibody, treating patients with advanced solid tumors and lymphoma
Cancer Immunology, Immunotherapy (2023)
-
A Clinical Approach to Existing and Emerging Therapeutics in Neuromyelitis Optica Spectrum Disorder
Current Neurology and Neuroscience Reports (2023)
-
Translational Approach for Predicting Human Pharmacokinetics of Engineered Therapeutic Monoclonal Antibodies with Increased FcRn-Binding Mutations
BioDrugs (2023)