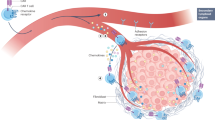
Abstract
The efficacy of chimeric antigen receptor (CAR) T cell therapy against poorly responding tumors can be enhanced by administering the cells in combination with immune checkpoint blockade inhibitors. Alternatively, the CAR construct has been engineered to coexpress factors that boost CAR-T cell function in the tumor microenvironment. We modified CAR-T cells to secrete PD-1-blocking single-chain variable fragments (scFv). These scFv-secreting CAR-T cells acted in both a paracrine and autocrine manner to improve the anti-tumor activity of CAR-T cells and bystander tumor-specific T cells in clinically relevant syngeneic and xenogeneic mouse models of PD-L1+ hematologic and solid tumors. The efficacy was similar to or better than that achieved by combination therapy with CAR-T cells and a checkpoint inhibitor. This approach may improve safety, as the secreted scFvs remained localized to the tumor, protecting CAR-T cells from PD-1 inhibition, which could potentially avoid toxicities associated with systemic checkpoint inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jackson, H.J., Rafiq, S. & Brentjens, R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Zhang, M. et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 7, 19 (2014).
Knutson, K.L. et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol. Immunother. 64, 1495–1504 (2015).
Erfani, N. et al. FoxP3+ regulatory T cells in peripheral blood of patients with epithelial ovarian cancer. Iran. J. Immunol. 11, 105–112 (2014).
Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009).
Noy, R. & Pollard, J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Abiko, K. et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112, 1501–1509 (2015).
Yeku, O.O., Purdon, T.J., Koneru, M., Spriggs, D. & Brentjens, R.J. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 7, 10541 (2017).
Frey, A.B. Suppression of T cell responses in the tumor microenvironment. Vaccine 33, 7393–7400 (2015).
Kershaw, M.H. et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Brahmer, J.R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Armand, P. Immune checkpoint blockade in hematologic malignancies. Blood 125, 3393–3400 (2015).
Rizvi, N.A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Tumeh, P.C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Cha, E. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 6, 238ra70 (2014).
Gajewski, T.F., Louahed, J. & Brichard, V.G. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 16, 399–403 (2010).
Ku, G.Y. et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 116, 1767–1775 (2010).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Curran, K.J. et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol. Ther. 23, 769–778 (2015).
Pegram, H.J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Koneru, M., Purdon, T.J., Spriggs, D., Koneru, S. & Brentjens, R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. OncoImmunology 4, e994446 (2015).
Pegram, H.J. et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia 29, 415–422 (2015).
Rafiq, S. et al. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms Tumor 1 antigen. Leukemia 31, 1788–1797 (2017).
Avanzi, M.P. et al. Engineered tumor-targeted t cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Reports 23, 2130–2141 (2018).
John, L.B. et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 19, 5636–5646 (2013).
Rosewell Shaw, A. et al. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol. Ther. 25, 2440–2451 (2017).
Chekmasova, A.A. et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin. Cancer Res. 16, 3594–3606 (2010).
Curran, M.A., Montalvo, W., Yagita, H. & Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 107, 4275–4280 (2010).
Santos, E.B. et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat. Med. 15, 338–344 (2009).
Suarez, E.R. et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 7, 34341–34355 (2016).
Prosser, M.E., Brown, C.E., Shami, A.F., Forman, S.J. & Jensen, M.C. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol. Immunol. 51, 263–272 (2012).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Michot, J.M. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016).
Grosso, J.F. et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117, 3383–3392 (2007).
Workman, C.J. et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 172, 5450–5455 (2004).
Sabatos, C.A. et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4, 1102–1110 (2003).
Hodi, F.S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Peterson, A.C., Russell, J.D., Bailey, D.J., Westphall, M.S. & Coon, J.J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell. Proteomics 11, 1475–1488 (2012).
Egertson, J.D., MacLean, B., Johnson, R., Xuan, Y. & MacCoss, M.J. Multiplexed peptide analysis using data-independent acquisition and Skyline. Nat. Protoc. 10, 887–903 (2015).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J.V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007).
Lee, A.Y. et al. Measurement of fractional synthetic rates of multiple protein analytes by triple quadrupole mass spectrometry. Clin. Chem. 58, 619–627 (2012).
Acknowledgements
We would like to acknowledge Y. Iragashi and A. Rookard for technical assistance with in vivo experiments and the MSKCC Molecular Cytogenetics Core and NIH Cancer Center support grant P30 CA008748 for karyotyping of the ID8 and SKOV3 cells. The authors thank the following for financial support: US National Institutes of Health grants 5 P01 CA190174-03 and 5 P50 CA192937-02 (R.J.B.), The Annual Terry Fox Run for Cancer Research organized by the Canada Club of New York (R.J.B.), Kate's Team (R.J.B.), Carson Family Charitable Trust (R.J.B.), the Leukemia & Lymphoma Society Specialized Center of Research Program (7014) (R.J.B.), William Lawrence and Blanche Hughes Foundation (R.J.B.), the ARD Foundation (R.J.B.), and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center (Innovations in the structures, functions and targets of monoclonal antibody-based drugs for cancer) (R.J.B.).
Author information
Authors and Affiliations
Contributions
S.R., O.O.Y., H.J.J. and R.J.B. designed the experiments, interpreted the results and wrote the manuscript. T.J.P., D.G.v.L., D.J.D., M.S., M.M.M., Z.L., P.W., S.Y. J.X. X.M., R.C.H. and C.L. designed, performed and/or analyzed the experiments. V.E.S. performed the statistical analysis.
Corresponding author
Ethics declarations
Competing interests
R.J.B. is a co-founder and receives royalties from Juno Therapeutics. R.J.B., S.R., H.J.J., O.Y. and C.L. have submitted a patent related to this work.
Integrated supplementary information
Supplementary Figure 1
(a) Entire western blot of analysis shown in Figure 1c on supernatant from equivalent numbers of viral packaging cells transduced to express the secretable scFv with the CAR, detected with anti-myc-tag antibody. Data shown is representative of 3 independent experiments. (b) Representative dot plot examples of flow cytometric data quantifying PD-1 detected on CAR-T cells in Figure 1g. Data shown is representative from 4 independent experiments.
Supplementary Figure 2 Detection of scFv secreted by CAR-T cells in vivo.
Entire western blot from Figure 2b analysis of in vivo secretion of RMP1-14 scFv, detected by harvesting ascites from CAR-T cell treated, tumor-bearing mice after immunoprecipitation with an anti-myc-tag antibody.
Supplementary Figure 3 Endogenous, CAR−-T cells extracted from C57BL/6 mice bearing B16-F10 mouse melanoma and treated with PD-1-blocking scFv CAR-T cells have enhanced activation and cytokine levels as compared to mice treated with second generation CAR-T cells.
(a-b) Representative plots demonstrating gating strategy utilized for flow cytometric quantification of CAR-T cells in Figure 2g. Data shown is representative of 2 independent experiments with 3 mice per condition, per experiment.
Supplementary Figure 4
(a) Entire western blot from Figure 3c of analysis on supernatant from equivalent numbers of 293-Glv9 packaging cells transduced to secrete scFvs with the 1928z CAR, stained with anti-HA antibody. Datan show is representative of 2 independent experiments. (b) Entire western blot from Figure 3f on supernatant from CAR-T cells stained with anti-HA mAb, demonstrating a ~30 kDa protein in the 1928z-E27 and 4H1128z-E27 T cells. Data shown is representative of 2 independent experiments. (c) Entire western blot from Figure 3g of 293-Glv9-PD-1+ cells incubated in supernatant (SN) from 1928z and 1928z-E27 T cells, stained with anti-HA mAb, showing a ~30 kDa protein in the PD-1+ cells incubated with supernatant from 1928z-E27. Data shown is representative of 2 independent experiments. (d) Representative dot plot from Figure 3h, showing decreased PD-1 detection by flow cytometry on 1928z-E27 and 4H1128z-E27 T cell, as compared to second-generation CAR-T cells. Data shown is representative of 5 independent donors.
Supplementary Figure 5 Western blot analysis of scFv binding to CAR− and CAR+ populations after co-culture.
Entire western blot from Figure 4f showing western blot analysis on flow sorted CAR+ and CAR−-T cell populations probed with anti-HA mAb.
Supplementary Figure 6 Quantification utilizing unique peptide sequences by liquid chromatography-tandem mass spectrometry (LC-MS/MS) of serum levels over time of E27 scFv and anti-human PD-1 mAb.
Peptide FGSNLESGIPAR (m/z = 624.3226) from the EH12/2H7 Ab. a) Skyline analysis to identify unique peptides in mAb but absent in the mouse or human proteome. The six y-type ions above or near the doubly charged precursor mass are shown. b) SDS-PAGE gel from EH12/2H7 AB. 6 μg/lane were loaded. Heavy chain and light chain were analyzed by data-dependent LC-MS/MS analysis as described. Light chain is shown labeled at band 2. c) High resolution HCD MS/MS spectra obtained on the (M+2H)2+ ions at 624.3226. Peptide FSGSNSGNTATLTISR (m/z = 806.8999) from the E27 scFv. d) Skyline analysis to identify unique peptides in the scFv but absent in the mouse or human proteome. The six y-type ions above or near the doubly charged precursor mass are shown. e) SDS-PAGE gel from E27 scFv. 6 μg/lane were loaded. ScFv was analyzed by data-dependent LC-MS/MS analysis as described. ScFv is shown labeled at band 2. f) High resolution HCD MS/MS spectra obtained on the(M+2H)2+ ions at 806.8999. Data shown is representative of 5 mice per group.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 909 kb)
Rights and permissions
About this article
Cite this article
Rafiq, S., Yeku, O., Jackson, H. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 36, 847–856 (2018). https://doi.org/10.1038/nbt.4195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.4195
This article is cited by
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases
Nature Biotechnology (2024)
-
Efficacy of programmed cell death 1 inhibitor maintenance after chimeric antigen receptor T cells in patients with relapsed/refractory B-cell non-Hodgkin-lymphoma
Cellular Oncology (2024)
-
Recent progress in chimeric antigen receptor therapy for acute myeloid leukemia
Annals of Hematology (2024)
-
CAR-T cell therapy: a game-changer in cancer treatment and beyond
Clinical and Translational Oncology (2024)