Abstract
The Wiskott–Aldrich syndrome protein (WASP) family of molecules integrates upstream signalling events with changes in the actin cytoskeleton. N-WASP has been implicated both in the formation of cell-surface projections (filopodia) required for cell movement and in the actin-based motility of intracellular pathogens. To examine N-WASP function we have used homologous recombination to inactivate the gene encoding murine N-WASP. Whereas N-WASP-deficient embryos survive beyond gastrulation and initiate organogenesis, they have marked developmental delay and die before embryonic day 12. N-WASP is not required for the actin-based movement of the intracellular pathogen Listeria but is absolutely required for the motility of Shigella and vaccinia virus. Despite these distinct defects in bacterial and viral motility, N-WASP-deficient fibroblasts spread by using lamellipodia and can protrude filopodia. These results imply a crucial and non-redundant role for N-WASP in murine embryogenesis and in the actin-based motility of certain pathogens but not in the general formation of actin-containing structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998).
Miki, H., Sasaki, T., Takai, Y. & Takenawa, T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391, 93–96 (1998).
Castellano, F. et al. Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr. Biol. 9, 351–360 (1999).
Derry, J. M., Ochs, H. D. & Francke, U. Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell 78, 635–644 (1994).
Miki, H., Miura, K. & Takenawa, T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 15, 5326–5335 (1996).
Suetsugu, S., Miki, H. & Takenawa, T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate wth the Arp2/3 complex. Biochem. Biophys. Res. Commun. 260, 296–302 (1999).
Bear, J. E., Rawls, J. F. & Saxe, C. L., 3rd. SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J. Cell Biol. 142, 1325–1335 (1998).
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998).
Yarar, D., To, W., Abo, A. & Welch, M. D. The Wiskott–Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 9, 555–558 (1999).
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999).
Machesky, L. M. & Insall, R. H. Signaling to actin dynamics. J. Cell Biol. 146, 267–272 (1999).
Higgs, H. N. & Pollard, T. D. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J. Biol. Chem. 274, 32531–32534 (1999).
Winter, D., Lechler, T. & Li, R. Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein. Curr. Biol. 9, 501–504 (1999).
Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, 1007–1011 (2000).
Kim, A. S., Kakalis, L. T., Abdul-Manan, N., Liu, G. A. & Rosen, M. K. Autoinhibition and activation mechanisms of the Wiskott–Aldrich syndrome protein. Nature 404, 151–158 (2000).
Higgs, H. N. & Pollard, T. D. Activation by Cdc42 and PIP2 of Wiskott–Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150, 1311–1320 (2000).
Rohatgi, R., Ho, H. Y. & Kirschner, M. W. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 150, 1299–1310 (2000).
Prehoda, K. E., Scott, J. A., Dyche Mullins, R. & Lim, W. A. Integration of multiple signals through cooperative regulation of the N-WASP–Arp2/3 complex. Science 290, 801–806 (2000).
Takenawa, T. & Miki, H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801–1809 (2001).
Finlay, B. B. & Cossart, P. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276, 718–725 (1997).
Dramsi, S. & Cossart, P. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell. Dev. Biol. 14, 137–166 (1998).
Suzuki, T., Miki, H., Takenawa, T. & Sasakawa, C. Neural Wiskott–Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17, 2767–2776 (1998).
Loisel, T. P., Boujemaa, R., Pantaloni, D. & Carlier, M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616 (1999).
Egile, C. et al. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146, 1319–1332 (1999).
Frischknecht, F. et al. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401, 926–929 (1999).
Bernardini, M. L., Mounier, J., d'Hauteville, H., Coquis-Rondon, M. & Sansonetti, P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl Acad. Sci. USA 86, 3867–3871 (1989).
Goldberg, M. B., Barzu, O., Parsot, C. & Sansonetti, P. J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 175, 2189–2196 (1993).
Welch, M. D., Rosenblatt, J., Skoble, J., Portnoy, D. A. & Mitchison, T. J. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281, 105–108 (1998).
Welch, M. D., Iwamatsu, A. & Mitchison, T. J. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature 385, 265–269 (1997).
May, R. C. et al. The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr. Biol. 9, 759–762 (1999).
Moreau, V. et al. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nature Cell Biol. 2, 441–448 (2000).
Frischknecht, F. & Way, M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell. Biol. 11, 30–38 (2001).
Sugihara, K. et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 17, 3427–3433 (1998).
Chen, F. et al. Cdc42 is required for PIP2-induced actin polymerization and early development but not for cell viability. Curr. Biol. 10, 758–765 (2000).
Takayama, H., Tanigawa, T., Tanaka, Y. & Kimura, G. Induction of fibronectin expression, actin cable formation, and entry into S phase following reexpression of T antigen in mouse macrophages transformed by the tsA640 mutant of SV40. J. Cell. Physiol. 128, 271–278 (1986).
Ulrich, R. G. & Quinlan, D. C. Cell surface and cytoskeletal interactions in a temperature-sensitive strain of SV40-transformed mouse fibroblasts. Cell Motil. 3, 553–565 (1983).
Kozma, R., Ahmed, S., Best, A. & Lim, L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15, 1942–1952 (1995).
Ramesh, N., Anton, I. M., Hartwig, J. H. & Geha, R. S. WIP, a protein associated with Wiskott–Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. USA 94, 14671–14676 (1997).
Anton, I. M., Lu, W., Mayer, B. J., Ramesh, N. & Geha, R. S. The Wiskott–Aldrich syndrome protein-interacting protein (WIP) binds to the adaptor protein Nck. J. Biol. Chem. 273, 20992–20995 (1998).
Martinez-Quiles, N. et al. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nature Cell Biol. 3, 484–491 (2001).
Lasko, M. et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93, 5860–5865 (1996).
Snapper, S. B. et al. Wiskott–Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9, 81–91 (1998).
Fukuoka, M., Miki, H. & Takenawa, T. Identification of N-WASP homologs in human and rat brain. Gene 196, 43–48 (1997).
Klein, C., Bueler, H. & Mulligan, R. C. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J. Exp. Med. 191, 1699–1708 (2000).
Ory, D. S., Neugeboren, B. A. & Mulligan, R. C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl Acad. Sci. USA 93, 11400–11406 (1996).
Hed, J. Methods for distinguishing ingested from adhering particles. Methods Enzymol. 132, 198–204 (1986).
Rottger, S., Frischknecht, F., Reckmann, I., Smith, G. L. & Way, M. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J. Virol. 73, 2863–2875 (1999).
Oaks, E. V., Wingfield, M. E. & Formal, S. B. Plaque formation by virulent Shigella flexneri. Infect. Immun. 48, 124–129 (1985).
Hartwig, J. et al. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell 82, 643–653 (1995).
Acknowledgements
We thank M. Way, H. Miki, T. Takenawa, R. Rohatgi, M. Kirshner and R. Xavier for reagents; Y. Fujiwara and S. Orkin for assistance with in situ hybridization experiments; Y. Ohta for microinjection expertise; M. Charles for assistance in Shigella infections; W. Swat and F. Chen for critical suggestions on the manuscript; and members of the Snapper, Alt and Rosen laboratories for continued support. This work was supported by grants from the National Institutes of Health (NIH) (to S.B.S., F.S.R., S.M.T. and F.W.A.), the Howard Hughes Medical Institute (to F.W.A.), the Massachusetts General Hospital NIH Center for the Study of Inflammatory Bowel Disease (to S.B.S.), and the 2nd Department of Internal Medicine, Nagasaki University School of Medicine (to F.T.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary figures
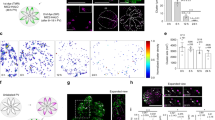
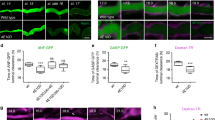
Figure S1 Nck and WIP are recruited to sites of actin assembly in Shigella- and vaccinia-infected SV-FLCs. (PDF 175 kb)
Figure S2 Neither Nck nor WIP is recruited to Shigella and vaccinia in the absence of N-WASP.
Rights and permissions
About this article
Cite this article
Snapper, S., Takeshima, F., Antón, I. et al. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol 3, 897–904 (2001). https://doi.org/10.1038/ncb1001-897
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1001-897
This article is cited by
-
Neural Wiskott-Aldrich syndrome protein (nWASP) is implicated in human lung cancer invasion
BMC Cancer (2017)
-
Conditional knock out of N-WASP in keratinocytes causes skin barrier defects and atopic dermatitis-like inflammation
Scientific Reports (2017)
-
The WASP-Arp2/3 complex signal cascade is involved in actin-dependent sperm nuclei migration during double fertilization in tobacco and maize
Scientific Reports (2017)
-
Isoform diversity in the Arp2/3 complex determines actin filament dynamics
Nature Cell Biology (2016)
-
How distinct Arp2/3 complex variants regulate actin filament assembly
Nature Cell Biology (2016)