Abstract
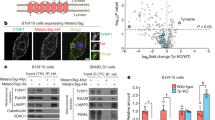
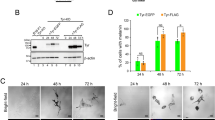
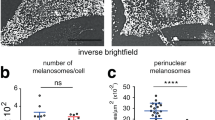
The synaptotagmin-like protein (Slp) family is implicated in regulating Rab27A-mediated membrane transport1,2,3, but how it might do this is unknown. Here we report that Slp2-a, a previously uncharacterized Rab27A-binding protein in melanocytes, controls melanosome distribution in the cell periphery and regulates the morphology of melanocytes. Slp2-a is the most abundantly expressed of the Slp- and Slac2-family proteins in melanocytes and colocalizes with Rab27A on melanosomes. Knockdown of endogenous Slp2-a protein by small-interfering RNAs (siRNAs) markedly reduced the number of melanosomes in the cell periphery of mouse melanocytes ('peripheral dilution'). Expression of siRNA-resistant Slp2-a (Slp2-aSR) rescued the peripheral dilution of melanosomes induced by Slp2-a siRNAs, but Slp2-aSR mutants, which failed to interact with either phospholipids or Rab27A, did not. Loss of Slp2-a protein also induced a change in melanocyte morphology, from their normal elongated shape to a more rounded shape, which depended on the phospholipid-binding activity of Slp2-a, but not on its Rab27A-binding activity. By contrast, knockdown of Slac2-a (also called melanophilin), another Rab27A-binding protein in melanocytes4,5, caused perinuclear aggregation of melanosomes alone without altering cell shape. These results reveal the differential and sequential roles of Rab27A-binding proteins in melanosome transport in melanocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kuroda, T. S., Fukuda, M., Ariga, H. & Mikoshiba, K. The Slp homology domain of synaptotagmin-like proteins 1–4 and Slac2 functions as a novel Rab27A binding domain. J. Biol. Chem. 277, 9212–9218 (2002).
Strom, M., Hume, A. N., Tarafder, A. K., Barkagianni, E. & Seabra, M. C. A family of Rab27-binding proteins: Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 277, 25423–25430 (2002).
Fukuda, M. Versatile role of Rab27 in membrane trafficking: Focus on the Rab27 effector families. J. Biochem. (in the press).
Hammer, J. A. III. & Wu, X. S. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr. Opin. Cell Biol. 14, 69–75 (2002).
Barral, D. C. & Seabra, M. C. The melanosome as a model to study organelle motility in mammals. Pigment Cell Res. 17, 111–118 (2004).
Marks, M. S. & Seabra, M. C. The melanosome: membrane dynamics in black and white. Nature Rev. Mol. Cell Biol. 2, 738–748 (2001).
Wu, X., Bowers, B., Rao, K., Wei, Q. & Hammer, J. A. III. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 143, 1899–1918 (1998).
Nascimento, A. A., Roland, J. T. & Gelfand, V. I. Pigment cells: a model for the study of organelle transport. Annu. Rev. Cell Dev. Biol. 19, 469–491 (2003).
Wu, X. et al. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 114, 1091–1100 (2001).
Hume, A. N. et al. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic 3, 193–202 (2002).
Wu, X. S., et al. Identification of an organelle receptor for myosin-Va. Nature Cell Biol. 4, 271–278 (2002).
Kuroda, T. S., Ariga, H. & Fukuda, M. The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes. Mol. Cell. Biol. 23, 5245–5255 (2003).
Fukuda, M., Kuroda, T. S. & Mikoshiba, K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 277, 12432–12436 (2002).
Provance, D. W. Jr, James, T. L. & Mercer, J. A. Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic 3, 124–132 (2002).
Ménasché, G. et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J. Clin. Invest. 112, 450–456 (2003).
Bahadoran, P. et al. Rab27a: A key to melanosome transport in human melanocytes. J. Cell Biol. 152, 843–850 (2001).
Hume, A. N. et al. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152, 795–808 (2001).
Wu, X., Wang, F., Rao, K., Sellers, J. R. & Hammer, J. A. III. Rab27a is an essential component of melanosome receptor for myosin Va. Mol. Biol. Cell 13, 1735–1749 (2002).
Bennett, D. C., Cooper, P. J. & Hart, I. R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 39, 414–418 (1987).
Raposo, G., Tenza, D., Murphy, D. M., Berson, J. F. & Marks, M. S. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 152, 809–824 (2001).
Wilson, S. M. et al. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl Acad. Sci. USA 97, 7933–7938 (2000).
Nalefski, E. A. & Falke, J. J. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 5, 2375–2390 (1996).
Fukuda, M., Kojima, T., Aruga, J., Niinobe, M. & Mikoshiba, K. Functional diversity of C2 domains of synaptotagmin family. Mutational analysis of inositol high polyphosphate binding domain. J. Biol. Chem. 270, 26523–26527 (1995).
Stinchcombe, J. C. et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152, 825–834 (2001).
Haddad, E. K., Wu, X., Hammer, J. A. III. & Henkart, P. A. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J. Cell Biol. 152, 835–842 (2001).
Trambas, C. M. & Griffiths, G. M. Delivering the kiss of death. Nature Immunol. 4, 399–403 (2003).
Shirakawa, R. et al. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J. Biol. Chem. 279, 10730–10737 (2004).
Feldmann, J. et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115, 461–473 (2003).
Imai, A., Yoshie, S., Nashida, T., Shimomura, H. & Fukuda, M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J. Cell Sci. 117, 1945–1953 (2004).
Fukuda M. RNA interference-mediated silencing of synaptotagmin IX, but not synaptotagmin I, inhibits dense-core vesicle exocytosis in PC12 cells. Biochem. J. 380, 875–879 (2004).
Acknowledgements
We thank D. C. Bennett (St. George's Hospital Medical School, London, UK) for the melan-a cell line, E. Kanno and Y. Ogata for expert technical assistance, and T. Itoh for valuable discussion. This work was supported in part by grants from the Ministry of Education, Culture, Sports and Technology of Japan (15689006 and 16044248 to M.F.). T.S.K. was supported by a Special Postdoctoral Researchers Program in RIKEN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Fig S1, Fig S2, Fig S3, Fig S4 (PDF 749 kb)
Rights and permissions
About this article
Cite this article
Kuroda, T., Fukuda, M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol 6, 1195–1203 (2004). https://doi.org/10.1038/ncb1197
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1197
This article is cited by
-
The nanoscale molecular morphology of docked exocytic dense-core vesicles in neuroendocrine cells
Nature Communications (2021)
-
Daphnane diterpenes inhibit the metastatic potential of B16F10 murine melanoma cells in vitro and in vivo
BMC Cancer (2018)
-
Methylquercetins stimulate melanin biosynthesis in a three-dimensional skin model
Journal of Natural Medicines (2018)
-
Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP
Scientific Reports (2015)