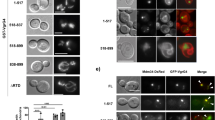
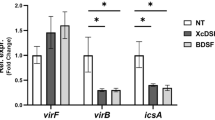
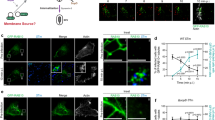
Abstract
Shigella use a special mechanism to invade epithelial cells called 'the trigger mechanism of entry'1,2,3, which allows epithelial cells to trap several bacteria simultaneously. On contact, Shigella deliver effectors into epithelial cells through the type III secretion system4,5,6. Here, we show that one of the effectors, IpgB1, has a pivotal role in producing membrane ruffles by exploiting the RhoG–ELMO–Dock180 pathway to stimulate Rac1 activity. Using pulldown assays, we identified engulfment and cell motility (ELMO) protein as the IpgB1 binding partner. IpgB1 colocalized with ELMO and Dock180 in membrane ruffles induced by Shigella. Shigella invasiveness and IpgB1-induced ruffles were less in ELMO- and Dock180-knockdown cells compared with wild-type cells. Membrane association of ELMO–Dock180 with ruffles were promoted when cells expressed an IpgB1–ELMO chimera, establishing that IpgB1 mimics the role of RhoG in producing membrane ruffles. Taken together, our findings show that IpgB1 mimicry is the key to invasion by Shigella.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Finlay, B. B. & Cossart, P. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276, 718–725 (1997).
Galan, J. E. & Collmer, A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328 (1999).
Cossart, P. & Sansonetti, P. J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304, 242–248 (2004).
Nhieu, G. T., Enninga, J., Sansonetti, P. & Grompone, G. Tyrosine kinase signaling and type III effectors orchestrating Shigella invasion. Curr. Opin. Microbiol. 8, 16–20 (2005).
Parsot, C. Shigella spp. and enteroinvasive Escherichia coli pathogenicity factors. FEMS Microbiol. Lett. 252, 11–18 (2005).
Ogawa, M. & Sasakawa, C. Intracellular survival of Shigella. Cell. Microbiol. 8, 177–184 (2006).
Alto, N. M. et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124, 133–145 (2006).
Ohya, K., Handa, Y., Ogawa, M., Suzuki, M. & Sasakawa, C. IpgB1 is a novel Shigella effector protein involved in bacterial invasion of host cells. J. Biol. Chem. 280, 24022–24034 (2005).
Gumienny, T. L. et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107, 27–41 (2001).
Brugnera, E. et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nature Cell Biol. 4, 574–582 (2002).
Lu, M. et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nature Struct. Mol. Biol. 11, 756–762 (2004).
Grimsley, C. M. et al. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J. Biol. Chem. 279, 6087–6097 (2004).
deBakker, C. D. et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 14, 2208–2216 (2004).
Katoh, H. & Negishi, M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 424, 461–464 (2003).
Bougneres, L. et al. Cortactin and Crk cooperate to trigger actin polymerization during Shigella invasion of epithelial cells. J. Cell Biol. 166, 225–235 (2004).
Yin, J. et al. Nuclear localization of the DOCK180/ELMO complex. Arch. Biochem. Biophys. 429, 23–29 (2004).
Dehio, C., Prevost, M. C. & Sansonetti, P. J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 14, 2471–2482 (1995).
Burton, E. A., Plattner, R. & Pendergast, A. M. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 22, 5471–5479 (2003).
Koleske, A. J. et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21, 1259–1272 (1998).
Hasegawa, H. et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell Biol. 16, 1770–1776 (1996).
Kiyokawa, E. et al. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 12, 3331–3336 (1998).
Katoh, H., Hiramoto, K. & Negishi, M. Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 119, 56–65 (2006).
Mounier, J. et al. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J. Cell Sci. 112, 2069–2080 (1999).
Patel, J. C. & Galan, J. E. Manipulation of the host actin cytoskeleton by Salmonella – all in the name of entry. Curr. Opin. Microbiol. 8, 10–15 (2005).
Okuda, J. et al. Shigella effector IpaH9.8 binds to a splicing factor U2AF(35) to modulate host immune responses. Biochem. Biophys. Res. Commun. 333, 531–539 (2005).
Sorensen, M. et al. Rapidly maturing red fluorescent protein variants with strongly enhanced brightness in bacteria. FEBS Lett. 552, 110–114 (2003).
Suzuki, T., Lett, M. C. & Sasakawa, C. Extracellular transport of VirG protein in Shigella. J. Biol. Chem. 270, 30874–30880 (1995).
Suzuki, M. et al. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 202, 1235–1247 (2005).
Yoshida, S. et al. Shigella deliver an effector protein to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization. EMBO J. 21, 2923–2935 (2002).
Baker, B. W., Boettiger, D., Spooncer, E. & Norton, J. D. Efficient retroviral-mediated gene transfer into human B lymphoblastoid cells expressing mouse ecotropic viral receptor. Nucleic Acids Res. 20, 5234 (1992).
Acknowledgements
We thank M. Matsuda for the Dock180 expression plasmid, D. Bumann for pDsRed.T3_S4T, and members of the Sasakawa laboratory for technical advice and experimental support. This work was supported by a grand-in-aid Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, and Technology (MEXT) and in part by the National BioResource Project in Japan (http://www.nbrp.jp/).
Author information
Authors and Affiliations
Contributions
Experimental work was performed by Y.H., K.O., H.I. and N.I. The project was planned by Y.H., M.S. and C.S. A.J.K and Y.F. provided materials.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4 and S5 (PDF 524 kb)
Supplementary Information
Supplementary Movie 1 (MOV 372 kb)
Supplementary Information
Supplementary Movie 2 (MOV 42 kb)
Supplementary Information
Supplementary Movie 3 (MOV 199 kb)
Rights and permissions
About this article
Cite this article
Handa, Y., Suzuki, M., Ohya, K. et al. Shigella IpgB1 promotes bacterial entry through the ELMO–Dock180 machinery. Nat Cell Biol 9, 121–128 (2007). https://doi.org/10.1038/ncb1526
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1526
This article is cited by
-
Molecular mechanisms of Shigella effector proteins: a common pathogen among diarrheic pediatric population
Molecular and Cellular Pediatrics (2022)
-
Role of ELMO1 in inflammation and cancer—clinical implications
Cellular Oncology (2022)
-
Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis
Scientific Reports (2020)
-
A systematic exploration of the interactions between bacterial effector proteins and host cell membranes
Nature Communications (2017)
-
The genomic signatures of Shigella evolution, adaptation and geographical spread
Nature Reviews Microbiology (2016)