Abstract
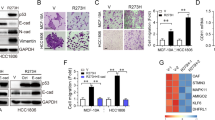
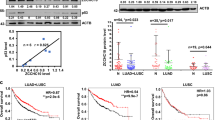
The tumour suppressor p53 is known to prevent cancer progression by inhibiting proliferation and inducing apoptosis of tumour cells. Slug, an invasion promoter, exerts its effects by repressing E-cadherin transcription. Here we show that wild-type p53 (wtp53) suppresses cancer invasion by inducing Slug degradation, whereas mutant p53 may stabilize Slug protein. In non-small-cell lung cancer (NSCLC), mutation of p53 correlates with low MDM2, high Slug and low E-cadherin expression. This expression profile is associated with poor overall survival and short metastasis-free survival in patients with NSCLC. wtp53 upregulates MDM2 and forms a wtp53–MDM2–Slug complex that facilitates MDM2-mediated Slug degradation. Downregulation of Slug by wtp53 or MDM2 enhances E-cadherin expression and represses cancer cell invasiveness. In contrast, mutant p53 inactivates Slug degradation and leads to Slug accumulation and increased cancer cell invasiveness. Our findings indicate that wtp53 and p53 mutants may differentially control cancer invasion and metastasis through the p53–MDM2–Slug pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
19 May 2009
In the version of this article initially published, The Myc-Ub label in Figure 3c was incorret. Arrowheads were missing in fig. 5d and 5e. A (+) sign was misplaced from the curve in Fig. 7e. These errors have been corrected in the HTML and PDF versions of the article.
References
Steeg, P. S. Tumor metastasis: mechanistic insights and clinical challenges. Nature Med. 12, 895–904 (2006).
Michael, D. & Oren, M. The p53–Mdm2 module and the ubiquitin system. Semin. Cancer Biol. 13, 49–58 (2003).
Vousden, K. H. & Lane, D. P. p53 in health and disease. Nature Rev. Mol. Cell Biol. 8, 275–283 (2007).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Soussi, T. & Beroud, C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nature Rev. Cancer 1, 233–240 (2001).
Yuan, A. et al. Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small-cell lung cancer. J. Clin. Oncol. 20, 900–910 (2002).
Bukholm, I. K., Nesland, J. M., Karesen, R., Jacobsen, U. & Borresen-Dale, A. L. Expression of E-cadherin and its relation to the p53 protein status in human breast carcinomas. Virchows Arch. 431, 317–321 (1997).
Hsiao, M. et al. Gain-of-function mutations of the p53 gene induce lymphohematopoietic metastatic potential and tissue invasiveness. Am. J. Pathol. 145, 702–714 (1994).
Lang, G. A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li–Fraumeni syndrome. Cell 119, 861–872 (2004).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li–Fraumeni syndrome. Cell 119, 847–860 (2004).
Mehta, S. A. et al. Negative regulation of chemokine receptor CXCR4 by tumor suppressor p53 in breast cancer cells: implications of p53 mutation or isoform expression on breast cancer cell invasion. Oncogene 26, 3329–3337 (2007).
Chen, Y. W. et al. Loss of p53 and Ink4a/Arf cooperate in a cell autonomous fashion to induce metastasis of hepatocellular carcinoma cells. Cancer Res. 67, 7589–7596 (2007).
Okawa, T. et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 21, 2788–2803 (2007).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev. Mol. Cell Biol. 7, 131–142 (2006).
Mareel, M. & Leroy, A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev. 83, 337–376 (2003).
Bremnes, R. M. et al. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J. Clin. Oncol. 20, 2417–2428 (2002).
Frixen, U. H. et al. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113, 173–185 (1991).
Handschuh, G. et al. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene 18, 4301–4312 (1999).
Perl, A. K., Wilgenbus, P., Dahl, U., Semb, H. & Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 (1998).
Hajra, K. M., Ji, X. & Fearon, E. R. Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene 18, 7274–7279 (1999).
Hennig, G. et al. Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene 11, 475–484 (1995).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nature Rev. Mol. Cell Biol. 3, 155–166 (2002).
Bolos, V. et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116, 499–511 (2003).
Hajra, K. M., Chen, D. Y. & Fearon, E. R. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 62, 1613–1618 (2002).
Shih, J. Y. et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin. Cancer Res. 11, 8070–8078 (2005).
Castro, A. C. et al. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J. Pathol. 211, 507–515 (2007).
Shioiri, M. et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br. J. Cancer 94, 1816–1822 (2006).
Martin, T. A., Goyal, A., Watkins, G. & Jiang, W. G. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann. Surg. Oncol. 12, 488–496 (2005).
Uchikado, Y. et al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin. Cancer Res. 11, 1174–1180 (2005).
Gupta, P. B. et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nature Genet. 37, 1047–1054 (2005).
Aylon, Y. & Oren, M. Living with p53, dying of p53. Cell 130, 597–600 (2007).
Manfredi, J. J. p53 and apoptosis: it's not just in the nucleus anymore. Mol. Cell 11, 552–554 (2003).
Kajita, M., McClinic, K. N. & Wade, P. A. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol. Cell Biol. 24, 7559–7566 (2004).
Lozano, G. The oncogenic roles of p53 mutants in mouse models. Curr. Opin. Genet. Dev. 17, 66–70 (2007).
Levrero, M. et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J. Cell Sci. 113, 1661–1670 (2000).
Havrilesky, L. et al. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 21, 3814–3825 (2003).
Cano, A. et al. Expression pattern of the cell adhesion molecules. E-cadherin, P-cadherin and α6 β4 integrin is altered in pre-malignant skin tumors of p53-deficient mice. Int. J. Cancer 65, 254–262 (1996).
Lewis, B. C. et al. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol. Cell Biol. 25, 1228–1237 (2005).
Derksen, P. W. et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (2006).
Bermejo-Rodriguez, C. et al. Mouse cDNA microarray analysis uncovers Slug targets in mouse embryonic fibroblasts. Genomics 87, 113–118 (2006).
Wu, W. S. et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123, 641–653 (2005).
Harada, J. et al. Requirement of the co-repressor homeodomain-interacting protein kinase 2 for ski-mediated inhibition of bone morphogenetic protein-induced transcriptional activation. J. Biol. Chem. 278, 38998–39005 (2003).
Mountain, C. F. Revisions in the international system for staging lung cancer. Chest 111, 1710–1717 (1997).
Travis, W., Brambilla, E., Müller-Hermelink, H. K. & Harris, C. C. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart (IARC Press, 2004).
Chang, Y. L. et al. Clonality and prognostic implications of p53 and epidermal growth factor receptor somatic aberrations in multiple primary lung cancers. Clin. Cancer Res. 13, 52–58 (2007).
Chu, Y. W. et al. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am. J. Respir. Cell Mol. Biol. 17, 353–360 (1997).
Acknowledgements
We thank H. K. Sytwu (Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan) for providing the plasmids for the lentivirus infection system, Pei-Fang Hung and Lu-Kai Wang for technical assistance, and Pei-Ying Lin for English language editing. This work was supported by the National Science Council and Department of Health, Executive Yuan of the Republic of China, through the National Research Program for Genomic Medicine Grants (DOH94-TD-G-111-020, DOH95-TD-G-111-010, DOH96-TD-G-111-009, DOH97-TD-G-111-018, DOH98-TD-G-111-007, NSC97-3112-B-002-033 and NSC97-2314-B-002-146-MY3). shRNA constructs were obtained from the National RNAi Core Facility at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, Taipei, Taiwan.
Author information
Authors and Affiliations
Contributions
T.-M.H. and P.-C.Y. directed the project and contributed equally to this work. S.-P.W. performed and analysed most of the experiments. W.-L.W., Y.-C.C., S.-H.K., S.-C.Y. and W.-K.C. provided reagents and materials, and performed data analysis. Y.-L.C., C.-T.W., A.Y., C.-W.L and K.-C.L. collected and analysed samples from lung cancer patients.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4262 kb)
Rights and permissions
About this article
Cite this article
Wang, SP., Wang, WL., Chang, YL. et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol 11, 694–704 (2009). https://doi.org/10.1038/ncb1875
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1875
This article is cited by
-
Harnessing function of EMT in cancer drug resistance: a metastasis regulator determines chemotherapy response
Cancer and Metastasis Reviews (2024)
-
Functional role and epithelial to mesenchymal transition of the miR-590-3p/MDM2 axis in hepatocellular carcinoma
BMC Cancer (2023)
-
FBXO28 suppresses liver cancer invasion and metastasis by promoting PKA-dependent SNAI2 degradation
Oncogene (2023)
-
Tp53 haploinsufficiency is involved in hotspot mutations and cytoskeletal remodeling in gefitinib-induced drug-resistant EGFRL858R-lung cancer mice
Cell Death Discovery (2023)
-
USP13 promotes breast cancer metastasis through FBXL14-induced Twist1 ubiquitination
Cellular Oncology (2023)