Abstract
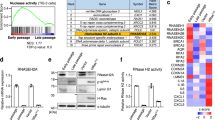
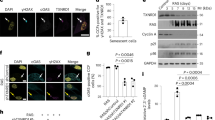
It is well known that aged or senescent cells develop a complex senescence-associated secretory phenotype (SASP), which is observed both in culture and in vivo. However, the mechanisms underlying the induction of the SASP are largely unknown. We demonstrate that retinoic-acid-inducible gene-I (RIG-I) is induced through the ataxia telangiectasia mutated–interferon regulatory factor 1 (ATM–IRF1) axis in senescent cells and that RIG-I signalling mediates the expression of two important mediators of inflammation, interleukin-6 (IL-6) and IL-8. Klotho has been associated with ageing. We show here that the intracellular, but not the secreted, form of klotho interacts with RIG-I and that this interaction inhibits RIG-I-induced expression of IL-6 and IL-8 both in vitro and in vivo. Our study uncovers a mechanism in which klotho functions as an anti-ageing factor through the suppression of RIG-I-mediated inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 March 2011
In the version of this article initially published online, a graph in Fig. 3b was mislabelled and there were errors in the text on pages 4 and 5 and in the figure legend for Fig. 4c.
References
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Wei, W., Hemmer, R. M. & Sedivy, J. M. Role of p14(ARF) in replicative and induced senescence of human fibroblasts. Mol. Cell Biol. 21, 6748–6757 (2001).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Shelton, D. N., Chang, E., Whittier, P. S., Choi, D. & Funk, W. D. Microarray analysis of replicative senescence. Curr. Biol. 9, 939–945 (1999).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Sebastian, T., Malik, R., Thomas, S., Sage, J. & Johnson, P. F. C/EBPβ cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 24, 3301–3312 (2005).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Nagai, T. et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 17, 50–52 (2003).
Nagai, R. et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell. Mol. Life Sci. 57, 738–746 (2000).
Saito, Y. et al. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun. 248, 324–329 (1998).
Matsumura, Y. et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 242, 626–630 (1998).
Shiraki-Iida, T. et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 424, 6–10 (1998).
Xiao, N.M., Zhang, Y.M., Zheng, Q. & Gu, J. Klotho is a serum factor related to human aging. Chin. Med. J. (Engl) 117, 742–747 (2004).
Tsujikawa, H., Kurotaki, Y., Fujimori, T., Fukuda, K. & Nabeshima, Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 (2003).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Chang, Q. et al. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 (2005).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Ikushima, M. et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem. Biophys. Res. Commun. 339, 827–832 (2006).
Mitobe, M. et al. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp. Nephrol. 101, e67–e74 (2005).
Yamamoto, M. et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 280, 38029–38034 (2005).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Kato, H. et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23, 19–28 (2005).
Xu, L. G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell. 19, 727–740 (2005).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122, 669–682 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Wang, J. et al. Retinoic acid-inducible gene-I mediates late phase induction of TNF-α by lipopolysaccharide. J. Immunol. 180, 8011–8019 (2008).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 β production. Nat. Immunol. 11, 63–69 (2010).
Kubota, K. et al. Retinoic acid-inducible gene-I is induced in gingival fibroblasts by lipopolysaccharide or poly IC: possible roles in interleukin-1β, -6 and -8 expression. Oral Microbiol. Immunol. 21, 399–406 (2006).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 (2009).
Pamment, J., Ramsay, E., Kelleher, M., Dornan, D. & Ball, K. L. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene 21, 7776–7785 (2002).
Kawaguchi, S. et al. Retinoic acid-inducible gene-I is constitutively expressed and involved in IFN-γ-stimulated CXCL9-11 production in intestinal epithelial cells. Immunol. Lett. 123, 9–13 (2009).
Imaizumi, T. et al. Retinoic acid-inducible gene-I (RIG-I) is induced by IFN-γ in human mesangial cells in culture: possible involvement of RIG-I in the inflammation in lupus nephritis. Lupus 19, 830–836 (2010).
Imaizumi, T. et al. Tumor-necrosis factor-α induces retinoic acid-inducible gene-I in rheumatoid fibroblast-like synoviocytes. Immunol. Lett. 122, 89–93 (2009).
Imaizumi, T. et al. Involvement of retinoic acid-inducible gene-I in inflammation of rheumatoid fibroblast-like synoviocytes. Clin. Exp. Immunol. 153, 240–244 (2008).
Maekawa, Y. et al. Klotho suppresses TNF-α-induced expression of adhesion molecules in the endothelium and attenuates NF-κB activation. Endocrine 35, 341–346 (2009).
Chen, C. D., Podvin, S., Gillespie, E., Leeman, S. E. & Abraham, C. R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl Acad. Sci. USA 104, 19796–19801 (2007).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Xu, L., Xiao, N., Liu, F., Ren, H. & Gu, J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc. Natl Acad. Sci. USA 106, 1530–1535 (2009).
Acknowledgements
We thank H. Shu, C. R. Abraham, M. Kuro-o, R. T. Moon and Y. Yang for plasmids, H. Deng and Y. Gao for irradiation, J. Luo and T. Tong for cell lines, Z. J. Chen and M. Kuro-o for knockout mice, M. Kuro-o for comments and J. L. Teeling for revising this paper. This work was supported by grants (2010CB911801 and 2011CB503904) from the National Basic Research Program, China.
Author information
Authors and Affiliations
Contributions
F.L. and S.W. designed and conducted experiments, analysed the data and wrote the manuscript. H.R. carried out experiments. J.G. designed experiments and analysed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 842 kb)
Rights and permissions
About this article
Cite this article
Liu, F., Wu, S., Ren, H. et al. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 13, 254–262 (2011). https://doi.org/10.1038/ncb2167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2167
This article is cited by
-
A prospective study of the association between serum klotho and mortality among adults with rheumatoid arthritis in the USA
Arthritis Research & Therapy (2023)
-
Prim-O-glucosylcimifugin ameliorates aging-impaired endogenous tendon regeneration by rejuvenating senescent tendon stem/progenitor cells
Bone Research (2023)
-
The role of α-klotho in human cancer: molecular and clinical aspects
Oncogene (2022)
-
A systematic review and meta-analysis demonstrating Klotho as an emerging exerkine
Scientific Reports (2022)
-
RIG-I acts as a tumor suppressor in melanoma via regulating the activation of the MKK/p38MAPK signaling pathway
Human Cell (2022)