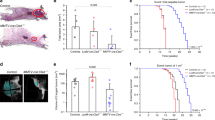
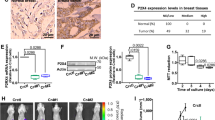
Abstract
It is well established that lysosomes play an active role during the execution of cell death1. A range of stimuli can lead to lysosomal membrane permeabilization (LMP), thus inducing programmed cell death without involvement of the classical apoptotic programme2,3. However, these lysosomal pathways of cell death have mostly been described in vitro or under pathological conditions4,5,6,7. Here we show that the physiological process of post-lactational regression of the mammary gland is accomplished through a non-classical, lysosomal-mediated pathway of cell death. We found that, during involution, lysosomes in the mammary epithelium undergo widespread LMP. Furthermore, although cell death through LMP is independent of executioner caspases 3, 6 and 7, it requires Stat3, which upregulates the expression of lysosomal proteases cathepsin B and L, while downregulating their endogenous inhibitor Spi2A (ref. 8). Our findings report a previously unknown, Stat3-regulated lysosomal-mediated pathway of cell death under physiological circumstances. We anticipate that these findings will be of major importance in the design of treatments for cancers such as breast, colon and liver, where cathepsins and Stat3 are commonly overexpressed and/or hyperactivated respectively1,9,10.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kroemer, G. & Jaattela, M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 5, 886–897 (2005).
Guicciardi, M. E., Leist, M. & Gores, G. J. Lysosomes in cell death. Oncogene 23, 2881–2890 (2004).
Hitomi, J. et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323 (2008).
Lockshin, R. A. & Zakeri, Z. Caspase-independent cell death? Oncogene 23, 2766–2773 (2004).
Nylandsted, J. et al. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res. 62, 7139–7142 (2002).
Luke, C. J. et al. An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell 130, 1108–1119 (2007).
Guicciardi, M. E., Miyoshi, H., Bronk, S. F. & Gores, G. J. Cathepsin B knockout mice are resistant to tumor necrosis factor-α-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am. J. Pathol. 159, 2045–2054 (2001).
Liu, N. et al. NF-κB protects from the lysosomal pathway of cell death. EMBO J. 22, 5313–5322 (2003).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Vasiljeva, O. et al. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene 27, 4191–4199 (2008).
Foghsgaard, L. et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153, 999–1010 (2001).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 (2000).
Kagedal, K., Zhao, M., Svensson, I. & Brunk, U. T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359, 335–343 (2001).
Nakayama, M. et al. Multiple pathways of TWEAK-induced cell death. J. Immunol. 168, 734–743 (2002).
Yamashima, T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62, 273–295 (2000).
Lascelles, A. K. & Lee, C. S. Lactation: A Comprehensive Treatise Vol. 4. (Academic, 1978).
Nguyen, A. V. & Pollard, J. W. Transforming growth factor β3 induces cell death during the first stage of mammary gland involution. Development 127, 3107–3118 (2000).
Kritikou, E. A. et al. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 130, 3459–3468 (2003).
Chapman, R. S. et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 13, 2604–2616 (1999).
Lund, L. R. et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 122, 181–193 (1996).
Thangaraju, M. et al. C/EBP δ is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 132, 4675–4685 (2005).
Baxter, F. O., Neoh, K. & Tevendale, M. C. The beginning of the end: death signaling in early involution. J. Mamm. Gland Biol. Neoplasia 12, 3–13 (2007).
Overholtzer, M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007).
Marti, A. et al. Mouse mammary gland involution is associated with cytochrome c release and caspase activation. Mech. Dev. 104, 89–98 (2001).
Zhou, Q. et al. Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics, and characterization of the caspase/p35 complex. Biochemistry 37, 10757–10765 (1998).
Fehrenbacher, N. et al. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res. 68, 6623–6633 (2008).
Zaragoza, R. et al. Nitration of cathepsin D enhances its proteolytic activity during mammary gland remodelling after lactation. Biochem. J. 419, 279–288 (2009).
Mohamed, M. M. & Sloane, B. F. Cysteine cathepsins: multifunctional enzymes in cancer. Nat. Rev. Cancer 6, 764–775 (2006).
Tiffen, P. G. et al. A dual role for oncostatin M signaling in the differentiation and death of mammary epithelial cells in vivo. Mol. Endocrinol. 22, 2677–2688 (2008).
Liu, N. et al. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat. Immunol. 5, 919–926 (2004).
Inglis, J. D. & Hill, R. E. The murine Spi-2 proteinase inhibitor locus: a multigene family with a hypervariable reactive site domain. EMBO J. 10, 255–261 (1991).
Clarkson, R. W., Wayland, M. T., Lee, J., Freeman, T. & Watson, C. J. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 6, R92–R109 (2004).
Clarkson, R. W. et al. NF-κB inhibits apoptosis in murine mammary epithelia. J. Biol. Chem. 275, 12737–12742 (2000).
Yu, Z., Zhang, W. & Kone, B. C. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor κB. Biochem. J. 367, 97–105 (2002).
Baxter, F. O. et al. IKKβ/2 induces TWEAK and apoptosis in mammary epithelial cells. Development 133, 3485–3494 (2006).
Reinhardt, T. A. & Lippolis, J. D. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochem. Biophys. Res. Commun. 378, 99–102 (2009).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Alonzi, T. et al. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol. Cell Biol. 21, 1621–1632 (2001).
Selbert, S. et al. Efficient BLG-Cre mediated gene deletion in the mammary gland. Transgenic Res. 7, 387–396 (1998).
Kuida, K. et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384, 368–372 (1996).
Lakhani, S. A. et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851 (2006).
Zheng, T. S. et al. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nature Med. 6, 1241–1247 (2000).
Warley, A., Powell, J. M. & Skepper, J. N. Capillary surface area is reduced and tissue thickness from capillaries to myocytes is increased in the left ventricle of streptozotocin-diabetic rats. Diabetologia 38, 413–421 (1995).
Khaled, W. T. et al. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development 134, 2739–2750 (2007).
Acknowledgements
We thank J. Skepper for help with the electron microscopy and B. Potter for histological preparations. We thank also P. Came for the TUNEL data and W. Khaled for discussions. This work has been supported by a PhD studentship from the Pathology Department, University of Cambridge (P.A.K.), Breast Cancer Campaign PhD studentship (A.D.S. and W.L.), Italian Cancer Research Association (V.P.) and Biotechnology and Biological Sciences Research Council grant no. BB/C006836/1 (R.W.E.C. and N.O.).
Author information
Authors and Affiliations
Contributions
P.A.K. carried out project design and all experiments, except those stated separately and wrote the manuscript; A.D.S. designed and carried out the in vitro studies; W.L. carried out studies on caspase-knockout mice; N.O. and R.W.E.C. generated the p35 transgenic mouse and provided data; B.K. carried out caspase studies; J.T. provided Stat3 inhibitor; V.P. provided Stat3fl/fl mice; R.A.F. provided all caspase-knockout mice; C.J.W. carried out project design and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 800 kb)
Rights and permissions
About this article
Cite this article
Kreuzaler, P., Staniszewska, A., Li, W. et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 13, 303–309 (2011). https://doi.org/10.1038/ncb2171
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2171
This article is cited by
-
Intracellular calcium links milk stasis to lysosome-dependent cell death during early mammary gland involution
Cellular and Molecular Life Sciences (2024)
-
Effects of sustained hyperprolactinemia in late gestation on the mammary parenchymal tissue transcriptome of gilts
BMC Genomics (2023)
-
SIM2s directed Parkin-mediated mitophagy promotes mammary epithelial cell differentiation
Cell Death & Differentiation (2023)
-
Fourteenth Annual ENBDC Workshop: Methods in Mammary Gland Biology and Breast Cancer
Journal of Mammary Gland Biology and Neoplasia (2023)
-
Fur removal promotes an earlier expression of involution-related genes in mammary gland of lactating mice
Journal of Comparative Physiology B (2023)