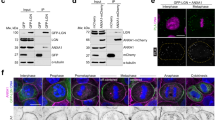
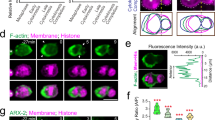
Abstract
Oriented mitosis is essential during tissue morphogenesis. The Wnt/planar cell polarity (Wnt/PCP) pathway orients mitosis in a number of developmental systems, including dorsal epiblast cell divisions along the animal–vegetal (A–V) axis during zebrafish gastrulation. How Wnt signalling orients the mitotic plane is, however, unknown. Here we show that, in dorsal epiblast cells, anthrax toxin receptor 2a (Antxr2a) accumulates in a polarized cortical cap, which is aligned with the embryonic A–V axis and forecasts the division plane. Filamentous actin (F-actin) also forms an A–V polarized cap, which depends on Wnt/PCP and its effectors RhoA and Rock2. Antxr2a is recruited to the cap by interacting with actin. Antxr2a also interacts with RhoA and together they activate the diaphanous-related formin zDia2. Mechanistically, Antxr2a functions as a Wnt-dependent polarized determinant, which, through the action of RhoA and zDia2, exerts torque on the spindle to align it with the A–V axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Castanon, I. & Gonzalez-Gaitan, M. Oriented cell division in vertebrate embryogenesis. Curr. Opin. Cell Biol. 23, 697–704 (2011).
Neumuller, R. A. & Knoblich, J. A. Dividing cellular asymmetry: Asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 23, 2675–2699 (2009).
Gonczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 (2008).
Gong, Y., Mo, C. & Fraser, S. E. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689–693 (2004).
Quesada-Hernandez, E. et al. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr. Biol. 20, 1966–1972 (2010).
Schlessinger, K., Hall, A. & Tolwinski, N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 23, 265–277 (2009).
Ridley, A. J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 (2006).
Riento, K. & Ridley, A. J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456 (2003).
Bartolini, F. & Gundersen, G. G. Formins and microtubules. Biochim. Biophys. Acta 1803, 164–173 (2010).
Chesarone, M. A., DuPage, A. G. & Goode, B. L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 11, 62–74 (2010).
Goode, B. L. & Eck, M. J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76, 593–627 (2007).
Lee, L., Klee, S. K., Evangelista, M., Boone, C. & Pellman, D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144, 947–961 (1999).
Miller, R. K., Matheos, D. & Rose, M. D. The cortical localization of the microtubule orientation protein, Kar9p, is dependent upon actin and proteins required for polarization. J. Cell Biol. 144, 963–975 (1999).
Azoury, J. et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 (2008).
Schuh, M. & Ellenberg, J. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 (2008).
Scobie, H. M., Rainey, G. J., Bradley, K. A. & Young, J. A. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl Acad. Sci. USA 100, 5170–5174 (2003).
Reeves, C. V., Dufraine, J., Young, J. A. & Kitajewski, J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene 29, 789–801 (2010).
Deuquet, J., Lausch, E., Superti-Furga, A. & van der Goot, F. G. The dark sides of capillary morphogenesis gene 2. EMBO J. 31, 3–13 (2012).
Macqueen, D. J. & Johnston, I. A. Evolution of follistatin in teleosts revealed through phylogenetic, genomic and expression analyses. Dev. Genes. Evol. 218, 1–14 (2008).
Cao, L. G. & Wang, Y. L. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J. Cell Biol. 111, 1905–1911 (1990).
Murthy, K. & Wadsworth, P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 15, 724–731 (2005).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Distel, M., Hocking, J. C., Volkmann, K. & Koster, R. W. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191, 875–890 (2010).
Bouzigues, C. & Dahan, M. Transient directed motions of GABA(A) receptors in growth cones detected by a speed correlation index. Biophys. J. 92, 654–660 (2007).
Fink, J. et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 13, 771–778 (2011).
Zhu, S., Liu, L., Korzh, V., Gong, Z. & Low, B. C. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling. Cell Signal 18, 359–372 (2006).
Sit, S. T. & Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 124, 679–683 (2011).
Lai, S. L. et al. Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements. PLoS One 3, e3439 (2008).
Li, F. & Higgs, H. N. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 (2003).
Liu, W. et al. Mechanism of activation of the Formin protein Daam1. Proc. Natl Acad. Sci. USA 105, 210–215 (2008).
Hancock, J. F. & Hall, A. A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 12, 1915–1921 (1993).
Matsui, T. et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 15, 2208–2216 (1996).
Marlow, F., Topczewski, J., Sepich, D. & Solnica-Krezel, L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 12, 876–884 (2002).
Rohde, L. A. & Heisenberg, C. P. Zebrafish gastrulation: cell movements, signals, and mechanisms. Int. Rev. Cytol. 261, 159–192 (2007).
Poincloux, R. et al. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc. Natl Acad. Sci. USA 108, 1943–1948 (2011).
Fukui, Y., Kitanishi-Yumura, T. & Yumura, S. Myosin II-independent F-actin flow contributes to cell locomotion in dictyostelium. J. Cell Sci. 112, 877–886 (1999).
Vallotton, P., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc. Natl Acad. Sci. USA 101, 9660–9665 (2004).
Hwang, E., Kusch, J., Barral, Y. & Huffaker, T. C. Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J. Cell Biol. 161, 483–488 (2003).
Liakopoulos, D., Kusch, J., Grava, S., Vogel, J. & Barral, Y. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell 112, 561–574 (2003).
Sagot, I., Klee, S. K. & Pellman, D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42–50 (2002).
Yang, H. C. & Pon, L. A. Actin cable dynamics in budding yeast. Proc. Natl Acad. Sci. USA 99, 751–756 (2002).
Ishizaki, T. et al. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3, 8–14 (2001).
Kiyomitsu, T. & Cheeseman, I. M. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat. Cell Biol. 14, 311–317 (2012).
Zeng, G. et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood 109, 1345–1352 (2007).
Cirone, P. et al. A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11, 347–360 (2008).
Westerfield, M. The zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) (Univ. Oregon Press, 2000).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dynam. 203, 253–310 (1995).
Abrami, L. et al. Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6. Cell Microbiol. 10, 2509–2519 (2008).
Montero, J. A. et al. Shield formation at the onset of zebrafish gastrulation. Development 132, 1187–1198 (2005).
Kimmel, C. B., Warga, R. M. & Kane, D. A. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development 120, 265–276 (1994).
Ulrich, F. et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell 9, 555–564 (2005).
O’Connor, M. N. et al. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood 113, 4754–4762 (2009).
Kilian, B. et al. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 120, 467–476 (2003).
Topczewski, J. et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell 1, 251–264 (2001).
Solnica-Krezel, L. et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 123, 67–80 (1996).
Schulte-Merker, S., van Eeden, F. J., Halpern, M. E., Kimmel, C. B. & Nusslein-Volhard, C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120, 1009–1015 (1994).
Akimenko, M. A., Ekker, M., Wegner, J., Lin, W. & Westerfield, M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J. Neurosci. 14, 3475–3486 (1994).
Thisse, C., Thisse, B., Halpern, M. E. & Postlethwait, J. H. Goosecoid expression in neurectoderm and mesendoderm is disrupted in zebrafish cyclops gastrulas. Dev. Biol. 164, 420–429 (1994).
Acknowledgements
We are grateful to B. Kunz and N. Zangger for generating the zAntxr2a construct for the pulldown, the phylogenetic tree and multiple sequence alignments, S. Abke, E. Paluch, C. Low, G. Weidinger, R. W. Köster, E. Kardash, L. Solnica-Krezel, A. J. Ridley, G. Davidson, S. Loubéry and O. Schaad, and the bioimaging and fish platforms for reagents and technical help and T. Hannich for critical reading. This work was supported by the SNSF, the Swiss SystemsX.ch initiative and LipidX-2008/011 (M.G-G. and F.G.v.d.G.), by the Fondation SANTE-Vaduz/Aide au Soutien des Nouvelles Thérapies (F.G.v.d.G.) and by the ERC, the NCCR Frontiers in Genetics and Chemical Biology programmes and the Polish–Swiss research program (M.G-G.).
Author information
Authors and Affiliations
Contributions
I.C. designed and carried out the experiments, generated and interpreted most of the data and prepared the manuscript. L.A. designed and carried out the immunoprecipitation experiments. L.H. designed the algorithm and simulation assays for the directed rotation experiments and C.P.H. supported experimentation and contributed to data interpretation. I.C., F.G.v.d.G. and M.G-G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2219 kb)
Supplementary Note
Supplementary Information (PDF 212 kb)
Supplementary Table
Supplementary Information (XLSX 58 kb)
Supplementary Movie 1
Supplementary Information (AVI 3482 kb)
Supplementary Movie 2
Supplementary Information (AVI 312 kb)
Supplementary Movie 3
Supplementary Information (AVI 2707 kb)
Supplementary Movie 4
Supplementary Information (AVI 452 kb)
Supplementary Movie 5
Supplementary Information (AVI 411 kb)
Supplementary Movie 6
Supplementary Information (AVI 920 kb)
Supplementary Movie 7
Supplementary Information (AVI 369 kb)
Rights and permissions
About this article
Cite this article
Castanon, I., Abrami, L., Holtzer, L. et al. Anthrax toxin receptor 2a controls mitotic spindle positioning. Nat Cell Biol 15, 28–39 (2013). https://doi.org/10.1038/ncb2632
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2632
This article is cited by
-
Capillary morphogenesis gene 2 (CMG2) mediates growth factor-induced angiogenesis by regulating endothelial cell chemotaxis
Angiogenesis (2022)
-
Wnt-controlled sphingolipids modulate Anthrax Toxin Receptor palmitoylation to regulate oriented mitosis in zebrafish
Nature Communications (2020)
-
Different mechanisms of two anti-anthrax protective antigen antibodies and function comparison between them
BMC Infectious Diseases (2019)
-
NDP52 tunes cortical actin interaction with astral microtubules for accurate spindle orientation
Cell Research (2019)
-
Up-regulated NRIP2 in colorectal cancer initiating cells modulates the Wnt pathway by targeting RORβ
Molecular Cancer (2017)