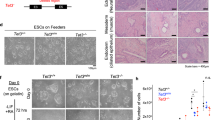
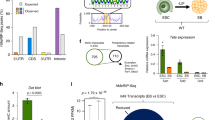
Abstract
How embryonic stem cells (ESCs) commit to specific cell lineages and yield all cell types of a fully formed organism remains a major question. ESC differentiation is accompanied by large-scale histone and DNA modifications, but the relations between these epigenetic categories are not understood. Here we demonstrate the interplay between the histone deacetylase sirtuin 6 (SIRT6) and the ten-eleven translocation enzymes (TETs). SIRT6 targets acetylated histone H3 at Lys 9 and 56 (H3K9ac and H3K56ac), while TETs convert 5-methylcytosine into 5-hydroxymethylcytosine (5hmC). ESCs derived from Sirt6 knockout (S6KO) mice are skewed towards neuroectoderm development. This phenotype involves derepression of OCT4, SOX2 and NANOG, which causes an upregulation of TET-dependent production of 5hmC. Genome-wide analysis revealed neural genes marked with 5hmC in S6KO ESCs, thereby implicating TET enzymes in the neuroectoderm-skewed differentiation phenotype. We demonstrate that SIRT6 functions as a chromatin regulator safeguarding the balance between pluripotency and differentiation through Tet-mediated production of 5hmC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Chen, T. & Dent, S. Y. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat. Rev. Genet. 15, 93–106 (2014).
Smith, Z. D. & Meissner, A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 14, 204–220 (2013).
Iyer, L. M., Tahiliani, M., Rao, A. & Aravind, L. Prediction of novel family of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle 11, 1698–1710 (2009).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
He, Y. F. et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333, 1303–1306 (2011).
Ito, S. et al. Tet proteins can convert 5-methycytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303 (2011).
Ficz, G. et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature 473, 398–402 (2011).
Williams, K. et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348 (2011).
Wu, H. et al. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 25, 679–684 (2011).
Kriaucionis, S. & Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930 (2009).
Ito, S. et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 (2010).
Ko, M. et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468, 839–843 (2010).
Szwagierczak, A., Bultmann, S., Schmidt, C. S., Spada, F. & Leonhardt, H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 38, e181 (2010).
Koh, K. P. et al. Tet1 and tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8, 200–213 (2011).
Xie, W. et al. Histone H3 lysine 56 acetylation is linked to the core transcriptional network in human embryonic stem cells. Mol. Cell 33, 417–427 (2009).
Yang, B., Zwaans, B. M. M., Eckersdoroff, M. & Lombard, D. B. The sirtuin Sirt6 deacetylates H3K56Ac in vivo to promote genomic stability. Cell Cycle 16, 2662–2663 (2009).
Michishita, E. et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human Sirt6. Cell Cycle 16, 2664–2666 (2009).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Zhong, L. et al. The histone deacetylase SIRT6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293 (2010).
Sebastián, C. et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 151, 1185–1199 (2012).
Toiber, D. et al. SIRT6 recruits SNF2H to DNA break sites, preventing instability through chromatin remodeling. Mol. Cell 51, 454–468 (2013).
Young, R. A. Control of the embryonic stem cell state. Cell 144, 940–954 (2011).
Thomson, M. et al. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell 145, 875–889 (2011).
Wu, Y. et al. Oct4 and the small molecule inhibitor, SC1, regulates Tet2 expression in mouse embryonic stem cells. Mol. Biol. Rep. 40, 2897–2906 (2013).
Pastor, W. A. et al. Genome-wide mapping of 5-hydroximethylcytosine in embryonic stem cells. Nature 473, 394–397 (2011).
Lienhard, M., Grimm, C., Morkel, M., Herwig, R. & Chavez, L. MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinformatics 30, 284–286 (2014).
Huang, Y. et al. Distinct roles of the methylcytosine oxidases Tet1 and Tet2 in mouse embryonic stem cells. Proc. Natl Acad. Sci. USA 111, 1361–1366 (2014).
Stadler, M. B. et al. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature 480, 490–495 (2011).
Lodato, M. A. et al. SOX2 co-occupies distal enhancer elements with distinct POU factors in ESCs and NPCs to specific cell state. PLoS Genet. 9, e1003288 (2013).
Ram, O. et al. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell 147, 1628–1639 (2011).
Epsztejn-Litman, S. et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat. Struct. Mol. Biol. 15, 1176–1183 (2008).
Li, J. Y. et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol. Cell. Biol. 27, 8748–8759 (2007).
Folmes, C. D. et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 14, 264–271 (2011).
Panopoulos, A. D. et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 22, 168–177 (2012).
Prigione, A. & Adjaye, J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int. J. Dev. Biol. 54, 1729–1741 (2010).
Varum, S. et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 6, e20914 (2011).
Zhou, W. et al. HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transitions. EMBO J. 31, 2103–2116 (2012).
Zhang, J., Nuebel, E., Daley, G. Q., Koehler, C. M. & Teitell, M. A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11, 589–595 (2012).
Spruijt, C. G. et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 152, 1146–1159 (2013).
Dawlaty, M. M. et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Cell 24, 310–323 (2013).
Bocker, M. T. et al. Hydroxylation of 5-methylcytosine by TET2 maintains the active state of the mammalian HOXA cluster. Nat. Commun. 3, 818 (2012).
Gomes, I. C., Acquarone, M., Maciel Rde, M., Erlich, R. B. & Rehen, S. K. Analysis of pluripotent stem cells by using cryosections of embryoid bodies. J. Vis. Exp. 46 (2010)10.3791/2344
Gaspar-Maia, A. et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature 460, 863–868 (2013).
Shen, Y. et al. A map of the cis-regulatory sequence in the mouse genome. Nature 488, 116–120 (2012).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Leinonen, R., Sugawara, H. & Shumway, M. The sequence read archive. Nucleic Acids Res. 39, 19–21 (2011).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memoryefficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. et al. The sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Garber, M. et al. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol. Cell 47, 810–822 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Ying, Q-L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 (2003).
Sommer, C. A. et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27, 543–549 (2009).
Gomes, P. N. et al. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev. 20, 601–612 (2006).
Stadtfeld, M. et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 465, 175–181 (2010).
Acknowledgements
This work was supported in part by NIH grants GM093072-01, DK088190-01A1 (R.M.), 5R01HD058013-05 (to K.H.) and HD065812, CA151535 (to A.R.). R.M. is the Kristine and Bob Higgins MGH Research Scholar, the Warshaw Institute Fellow, and a Howard Goodman Awardee. L.C. was the recipient of a Feodor Lynen Research Fellowship from the Alexander von Humboldt Foundation. Y.H. was supported by a postdoctoral fellowship from the Leukemia and Lymphoma Society. C.A.S. is the recipient of the Evans Center Fellow Award. A.Gladden and A.Goren were the recipient of the Broad Institute SPARC (Scientific Projects to Accelerate Research and Collaboration) program. We thank O. Bar-Nur and S. Cheloufi for advice on the reprogramming of neural progenitor cells, and E. Kelliher for setting up the automation of the ChIP-Seq experiments.
Author information
Authors and Affiliations
Contributions
J-P.E. conceived and designed most of the experiments, collected and analysed data, and wrote the manuscript; L.C., M.L. and K.N.R. performed computational analyses; Y.H. performed ChIP-Seq experiments; J.C. performed human ESC experiments; C.A.S. and G.M. performed iPSC experiments; B.M-P. performed and analysed immunofluorescence experiments; R.M.W. performed neurogenesis and immunofluorescence experiments; A.Gladden performed ChIP-Seq experiments; S.K. performed cloning of human SIRT6 expression system; D.M.S. performed in vitro experiments; S.R. supervised computational analyses; K.H. supervised the human ESC experiments; A.Goren conceived, supervised and analysed all the ChIP-Seq experiments and their computational analysis; A.R. supervised computational analyses, 5hMeC Chip-Seq experiments and edited the manuscript; R.M. conceived and supervised the study, analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Gene expression linked to the skewed phenotype towards neuroectoderm in S6KO EBs, Related to Fig. 1.
(A) Immunofluorescence of EBs derived from WT and S6KO iPSCs (129 mouse strain) for Gfap (green). Scale bar, 500 μm. (B) Gene expression of trophectoderm and neuroectoderm genes in WT versus S6KO EBs. qRT-PCR data is expressed relative to WT EBs. Data is expressed relative to WT values. The data are n = 3 experimental replicates (from independent RNA preparations). Values are mean ± s.e.m. ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗∗P < 0.00001, by t-test analysis. (C) Immunofluorescence of in vitro generated neurons from WT and S6KO EBs for β-III Tubulin (red). Undifferentiated neurons are visualized with dapi staining (blue). This is a representative of n = 3 experimental replicates (independent experiments). Scale bar, 100 μm. (D) Intensity of immunofluorescence of images from n = 3 experimental replicates (independent cell preparations) was analyzed by Image J. Data was normalized to WT values and represented as mean ± s.e.m. ∗∗P < 0.01, by t-test analysis. (E) Western blots showing expression of Nestin and Tet2 in WT versus S6KO EBs cultured under regular EB-medium. Expression of Sirt6 and its target H3K56ac are also shown. (F) Gene expression of trophectoderm genes in WT versus S6KO ESCs. qRT-PCR data is expressed relative to WT EBs. Data is expressed relative to WT values. The data are n = 3 experimental replicates (from independent RNA preparations). Values are mean ± s.e.m. ∗∗∗P < 0.001, ∗∗∗∗∗P < 0.00001, by t-test analysis. (G) Inability to silence expression of Sox2 and Nanog upon retinoic acid-induced differentiation in S6KO ESCs. Western blot analysis showing the expression of core pluripotent proteins in WT versus S6KO ESCs during a time-course retinoic acid-induced differentiation assay. Molecular weight markers are indicated. (H) Levels of H3K9ac and H3K56ac in S6KO versus WT ESCs after retinoic acid (RA)-mediated differentiation. ChIP-Seq binding profiles of the histone marks H3K56ac and H3K9ac on Oct4, Sox2 and Nanog genes in WT versus S6KO ESCs after retinoic acid-mediated differentiation. Images were created with the Integrative Genomic Viewer (IGV)51. Data are normalized to total counts, and the scale range is 0.0–1.0. The data on panels (B) and (F) are n = 3 experimental replicates, values are mean ± s.e.m. ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗∗P < 0.00001 by t-test throughout the figure.
Supplementary Figure 2 The upregulated expression of neuronal differentiation related-genes exhibiting a 5hmC gain in S6KO over WT ESCs is rescued upon Tet knockdown, Related to Figures 5 and 6.
Gene expression analysis by qRT-PCR is expressed relative to WT ESCs. Data are n = 3 experimental replicates (independent RNA preparations), values are mean ± s.e.m. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001,∗∗∗∗∗∗∗∗∗P < 0.00000001, by t-test throughout the figure.
Supplementary Figure 3 The upregulated expression of neuronal differentiation related-genes exhibiting a 5hmC gain in S6KO over WT ESCs is rescued upon Tet knockdown, Related to Figures 5 and 6.
Gene expression analysis by qRT-PCR is expressed relative to WT ESCs. Data are n = 3 experimental replicates (independent RNA preparations), values are mean ± s.e.m. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001,∗∗∗∗∗∗∗∗∗P < 0.00000001, by t-test throughout the figure.
Supplementary Figure 4
(A) The upregulated expression of neuronal differentiation related-genes exhibiting a 5hmC gain in S6KO over WT ESCs is rescued upon Tet knockdown, Related to Figures 5 and 6. Gene expression analysis by qRT-PCR is expressed relative to WT ESCs. Data are n = 3 experimental replicates (independent RNA preparations), values are mean ± s.e.m. ∗P < 0.05,∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, by t-test throughout the figure. (B) Sirt6 is recruited to the core pluripotent factors in human ESCs, Related to Figure 8. Genome wide Sirt6 ChIP-Seq data originated by Ram and colleagues30 was used to determine recruitment of human Sirt6 (hSirt6) to Oct4 and Sox2. (C) A small but significant peak for hSirt6 binding is apparent in Tet1, but not Tet2 gene. Statistically enriched hSirt6 peaks are shown as red boxes. Histone marks (H3K4me1, H3K4me2 and H3K36me3) and p300 signals are also shown for reference. Supplementary Table S10 shows all the genomic regions targeted by hSirt6 analysed using the same approach as in Ram and colleagues30. The accession number for these data can be found at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32509.
Supplementary Figure 5
(A) Pdk1 knockdown does not rescue the differentiation phenotype in S6KO EBs, Related to Figure 3. EBs derived from WT and S6KO ESCs (129 mouse strain). Scale bar, 500 μm. Pictures were taken at days 4 and 6 during EB formation. Data are representative of n = 3 experimental replicates. (B) Elevated levels of 5caC in S6KO versus WT ESCs. Related to Figure 4. Global 5caC levels assayed by slot blot analysis in ESCs. (C) Graph showing densitometric quantification of 5caC levels at each concentration of genomic DNA from panel (B). Data are representative of n = 2 experimental replicates. (D) Tet downregulation rescues high levels of 5caC in S6KO versus WT ESCs. Global 5caC levels assayed by slot blot analysis in Tet knockdown ESCs. (E) Graph showing densitometric quantification of 5caC levels at each concentration of genomic DNA from panel (D). Data are representative of n = 2 experimental replicates. (F) Increase efficiency of somatic cell reprogramming in S6KO versus WT NPCs. iPSC colony formation assay measured with alkaline phosphatase. (G) Graph showing iPSC colonies from each genotype. (H) Graph showing the average of WT (n = 3) versus S6KO (n = 5) NPCs reprogrammed into iPSCs. Colonies were quantified by image J, and values are mean ± s.e.m. ∗P < 0.05, by t-test analysis.
Supplementary Figure 6 Ectopic expression of human Sirt6 rescues the differentiation phenotype of S6KO EBs.
(A) Western blot analysis showing expression of endogenous Sirt6 and ectopic hSirt6. Total histone H3 is shown as loading control. (B) Embryoid bodies grown till day 10. Genotypes are indicated. Scale bar, 500 μm. (C) Gene expression analysis by qRT-PCR is expressed relative to WT ESCs. Data are n = 3 experimental replicates (independent RNA preparations), values are mean ± s.e.m. ∗P < 0.05, ∗∗P < 0.01, by t-test analysis.
Supplementary Figure 7 Characterization of genomic regions with changes of H3K9ac and H3K56ac in S6KO versus WT ESCs, before and after retinoic acid (RA)-mediated differentiation.
MEDIPS software was used to find regions with differential histones H3K9ac and H3K56ac in 500 base windows with P < 0.001 and these regions with gains and losses upon Sirt6 KO were mapped to promoters, gene bodies, CpG islands, enhancers, and super-enhancers. (A) Genomic positions of H3K9ac gains and losses in mouse ESCs. (B) Genomic positions of H3K9ac gains and losses in RA-differentiated mouse ESCs. (C) Genomic positions of H3K56ac gains and losses in mouse ESCs. (D) Genomic positions of H3K56ac gains and losses in RA-differentiated mouse ESCs.
Supplementary Figure 8 Enrichment of 5hmC does not correlate with H3K9ac and/or H3K56ac in S6KO versus WT ESCs.
(A) Heat map plot of regions of H3K56ac gains found with MEDIPS software in S6KO over WT in mouse ESCs show average profile for a ±3kb band centered around the 18722 regions with H3K56ac gain (P < 0.001) for the factors 5hmC, H3K56ac, H3K9ac, Sirt6, and Sox2 in WT and S6KO ESCs. (B) Enrichment line plot of average profile for regions of H3K56ac gain in S6KO over WT in mouse ESCs (n = 18,722) for the data on panel (A). Semi-transparent band behind line shows standard error of the mean for each average profile. (C) Heat map plot of regions of H3K9ac gains found with MEDIPS software in S6KO over WT mouse ESCs show average profile for a ±3 kb band centered around the 39095 regions with H3K9ac gain (P < 0.001) for the factors 5hmC, H3K56ac, H3K9ac, Sirt6, and Sox2 in WT and S6KO ESCs. (D) Enrichment line plot of average profile for regions of H3K9ac gain in S6KO over WT in mouse ES cells (n = 39095) for the data on panel (C). Semi-transparent band behind line shows standard error of the mean for each average profile.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4906 kb)
Supplementary Table 1
Supplementary Information (XLSX 486 kb)
Supplementary Table 2
Supplementary Information (XLSX 300 kb)
Supplementary Table 3
Supplementary Information (XLSX 12 kb)
Supplementary Table 4
Supplementary Information (XLSX 534 kb)
Supplementary Table 5
Supplementary Information (XLSX 371 kb)
Supplementary Table 6
Supplementary Information (XLSX 678 kb)
Supplementary Table 7
Supplementary Information (XLSX 267 kb)
Supplementary Table 8
Supplementary Information (XLSX 305 kb)
Supplementary Table 9
Supplementary Information (XLSX 131 kb)
Supplementary Table 10
Supplementary Information (XLSX 111 kb)
Supplementary Table 11
Supplementary Information (XLSX 40 kb)
Rights and permissions
About this article
Cite this article
Etchegaray, JP., Chavez, L., Huang, Y. et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat Cell Biol 17, 545–557 (2015). https://doi.org/10.1038/ncb3147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3147
This article is cited by
-
SIRT6 is a key regulator of mitochondrial function in the brain
Cell Death & Disease (2023)
-
Nuclear localization of mitochondrial TCA cycle enzymes modulates pluripotency via histone acetylation
Nature Communications (2022)
-
Modulation of cellular processes by histone and non-histone protein acetylation
Nature Reviews Molecular Cell Biology (2022)
-
Multiomics interrogation into HBV (Hepatitis B virus)-host interaction reveals novel coding potential in human genome, and identifies canonical and non-canonical proteins as host restriction factors against HBV
Cell Discovery (2021)
-
Sirt6 opposes glycochenodeoxycholate-induced apoptosis of biliary epithelial cells through the AMPK/PGC-1α pathway
Cell & Bioscience (2020)