Abstract
Somatic cells can be reprogrammed to a pluripotent state by nuclear transfer into oocytes, yet developmental arrest often occurs. While incomplete transcriptional reprogramming is known to cause developmental failure, reprogramming also involves concurrent changes in cell cycle progression and nuclear structure. Here we study cellular reprogramming events in human and mouse nuclear transfer embryos prior to embryonic genome activation. We show that genetic instability marked by frequent chromosome segregation errors and DNA damage arise prior to, and independent of, transcriptional activity. These errors occur following transition through DNA replication and are repaired by BRCA1. In the absence of mitotic nuclear remodelling, DNA replication is delayed and errors are exacerbated in subsequent mitosis. These results demonstrate that independent of gene expression, cell-type-specific features of cell cycle progression constitute a barrier sufficient to prevent the transition from one cell type to another during reprogramming.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Ladewig, J., Koch, P. & Brustle, O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat. Rev. Mol. Cell Biol. 14, 225–236 (2013).
Mikkelsen, T. S. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008).
Noggle, S. et al. Human oocytes reprogram somatic cells to a pluripotent state. Nature 478, 70–75 (2011).
Yamada, M. et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature 510, 533–536 (2014).
Chung, Y. G. et al. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell 14, 777–780 (2014).
Ma, H. et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 524, 234–238 (2015).
Paull, D. et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 493, 632–637 (2013).
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J. & Campbell, K. H. Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813 (1997).
Fairman, M. P. & Stillman, B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 7, 1211–1218 (1988).
Terashita, Y. et al. Latrunculin a treatment prevents abnormal chromosome segregation for successful development of cloned embryos. PLoS ONE 8, e78380 (2013).
Crasta, K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58 (2012).
Flach, G., Johnson, M. H., Braude, P. R., Taylor, R. A. & Bolton, V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1, 681–686 (1982).
Rambhatla, L. & Latham, K. E. Strain-specific progression of α-amanitin-treated mouse embryos beyond the two-cell stage. Mol. Reprod. Dev. 41, 16–19 (1995).
Burrell, R. A. et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 494, 492–496 (2013).
Chan, K. L., Palmai-Pallag, T., Ying, S. & Hickson, I. D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 11, 753–760 (2009).
Kishigami, S. et al. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 340, 183–189 (2006).
Rybouchkin, A., Kato, Y. & Tsunoda, Y. Role of histone acetylation in reprogramming of somatic nuclei following nuclear transfer. Biol. Reprod. 74, 1083–1089 (2006).
Bui, H. T. et al. Effect of trichostatin A on chromatin remodeling, histone modifications, DNA replication, and transcriptional activity in cloned mouse embryos. Biol. Reprod. 83, 454–463 (2010).
Egli, D., Rosains, J., Birkhoff, G. & Eggan, K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature 447, 679–685 (2007).
Kang, E. et al. Nuclear reprogramming by interphase cytoplasm of two-cell mouse embryos. Nature 509, 101–104 (2014).
Helmrich, A., Ballarino, M. & Tora, L. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol. Cell 44, 966–977 (2011).
Mellon, I. & Hanawalt, P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature 342, 95–98 (1989).
Shakya, R. et al. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science 334, 525–528 (2011).
Di Berardino, M. A. & King, T. J. Development and cellular differentiation of neural nuclear-transplants of known karyotype. Dev. Biol. 15, 102–128 (1967).
Mizutani, E. et al. Abnormal chromosome segregation at early cleavage is a major cause of the full-term developmental failure of mouse clones. Dev. Biol. 364, 56–65 (2012).
Ryba, T. et al. Replication timing: a fingerprint for cell identity and pluripotency. PLoS Comput. Biol. 7, e1002225 (2011).
Shufaro, Y. et al. Reprogramming of DNA replication timing. Stem Cells 28, 443–449 (2010).
Hiratani, I. et al. Genome-wide dynamics of replication timing revealed by in vitro models of mouse embryogenesis. Genome Res. 20, 155–169 (2010).
Pope, B. D. et al. Topologically associating domains are stable units of replication-timing regulation. Nature 515, 402–405 (2014).
Naumova, N. et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013).
Lemaitre, J. M., Danis, E., Pasero, P., Vassetzky, Y. & Mechali, M. Mitotic remodeling of the replicon and chromosome structure. Cell 123, 787–801 (2005).
Waddington, C. H. The epigenotype. Int. J. Epidemiol. 41, 10–13 (2012).
Bortvin, A. et al. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 130, 1673–1680 (2003).
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012).
Polo, J. M. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632 (2012).
Marion, R. M. et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 (2009).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460, 1132–1135 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Gonzalez, F. et al. Homologous recombination DNA repair genes play a critical role in reprogramming to a pluripotent state. Cell Rep. 3, 651–660 (2013).
Muller, L. U. et al. Overcoming reprogramming resistance of Fanconi anemia cells. Blood 119, 5449–5457 (2012).
Pathania, S. et al. BRCA1 haploinsufficiency for replication stress suppression in primary cells. Nat. Commun. 5, 5496 (2014).
Egli, D. & Le Bin, G. C. Tying replication to cell identity. Nat. Rev. Mol. Cell Biol. 14, 326 (2013).
Chia, G. & Egli, D. Connecting the cell cycle with cellular identity. Cell. Reprogram. 15, 356–366 (2013).
Dominguez-Sola, D. et al. Non-transcriptional control of DNA replication by c-Myc. Nature 448, 445–451 (2007).
Treff, N. R., Su, J., Tao, X., Levy, B. & Scott, R. T. Jr Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil. Steril. 94, 2017–2021 (2010).
Sakaue-Sawano, A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008).
Hogan, B., Costantini, F. & Lacy, E. Manipulating the Mouse Embryo: A Laboratory Manual Vol. 500 (Cold Spring Harbor Laboratory Press, 1994).
Acknowledgements
This research was supported by the New York Stem Cell Foundation (NYSCF) and a New York State Stem Cell Science (NYSTEM) IIRP Award no. C026184 and the John M. Driscoll, Jr, M. D. Children’s Fund. D.E. is a NYSCF-Robertson Investigator. We thank W. Kearns (Shady Grove Center for Preimplantation Genetic Diagnosis) for initial help with karyotyping. G.C. is supported by the Agency for Science, Technology and Research (A ∗ STAR) International Fellowship. We thank A. Ciccia and W.-W. Tee for critical comments on the manuscript.
Author information
Authors and Affiliations
Contributions
G.C. and D.E. designed and performed the experiments, analysed the data and wrote the manuscript with input from all authors. D.E. conceived and supervised the studies and performed NT. J.A. and B.D.B. provided T cells. N.T. performed karyotype analysis of blastomeres. D.B. and R.B. provided Brca1 mutant mice and helped with data interpretation and manuscript writing. M.V.S. was involved in all aspects of oocyte donation, including consent and oocyte retrieval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
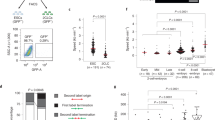
Supplementary Figure 1 Genetic instability in human nuclear transfer embryos.
(a) Normal chromosome segregation at the second meiotic division upon transfer of a somatic cell genome. (b–d) Anaphase at the first mitosis. b) The arrow points to a chromosome fragment. (c) The arrows point the direction of chromosome movement. Note the splitup of chromosomes into different groups, and a chromosome fragment (arrowhead). n = 142 (IVF), 138 (NT), 111 (NT, caffeine pulse) embryos. P = 0.0000 (Student’s t-test). (d) lagging chromosomes occurring in pairs (arrows). (e) A single confocal section of the cell shown in (d). Note the indentation (arrowhead) in the anaphase chromosomes, likely as a result of strain from a resolving chromosome bridge (arrow). (f) Formation of nuclei at telophase of the first mitosis. Note the formation of multiple nuclei. (g) 6-cell NT embryo with four blastomeres (one not visible) in mitosis on day 4 after transfer. Arrows point to the metaphase plate, arrowheads to chromosomes that fail to integrate. (h) Blastomere arrested in mitosis. Abnormal chromosome condensation prevents the formation of a metaphase plate. DNA damage is evident from phosphorylated γH2AX staining. The three channels were shifted relative to each other for visibility. (i,j) Examples of multinucleation in blastomeres. pH3 indicates phosphorylation on serine 10 of histone H3. (k) Another example of an embryo arrested with DNA damage, at entry into mitosis. (l,m) RPA foci in an interphase nucleus, and centromere staining. (n–p) Examples of RPA and phosphorylated γH2AX in NT blastomeres. Scale bar: 10 μm.
Supplementary Figure 2
SNP array analysis of copy number and heterozygosity in NT blastomeres. Each of the four clusters is derived from a different embryo. Only cells with successful biopsy and array analysis are shown.
Supplementary Figure 3 Different types of mitotic chromosomal defects in nuclear transfer embryos.
(a) Immunostaining of NT embryos at the first mitosis reconstituted using different donor cell types (ES cells, T-cells and cumulus cells). (b) Percentage of embryos (parthenotes and NT embryos), activated with either latrunculin A or cytochalasin B, with chromosomal segregation defects at the first mitosis. n = 64 (Parthenotes, Latrunculin A), 202 (Parthenotes, Cytochalasin B), 170 (NT, Latrunculin A) and 129 (NT, Cytochalasin B) embryos. Scale bar: 10 μm.
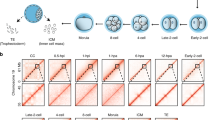
Supplementary Figure 4 Cell cycle of somatic donor cells.
Cell cycle profile by flow cytometry of naïve T-cells used for transfer. Note that most cells are in G1 of the cell cycle.
Supplementary Figure 5 DNA damage is reduced in G2, following exit from S-phase, and is independent of transcription.
NT embryos and parthenotes were incubated in EdU for 1 h at the following time points (17 h, 22 h and 28h postactivation). Bars represent the mean percentage of NT embryos that are undergoing DNA replication (EdU positive) at the respective time points. n = 12 (P, 17h), 13 (NT, 17h), 12 (P, 22h), 12 (NT, 22h), 5 (P, 28h) and 12 (NT, 28h) embryos.] b) DNA damage (no. of γH2AX) in NT embryos and parthenotes at the respective time points in a. Line represents mean number of foci in n = 12 (P, 17h), 13 (NT, 17h), 12 (P, 22h), 12 (NT, 22 h), 5 (P, 28h) and 12 (NT, 28h) number of embryos. ∗∗P < 0.01; ns = not significant (Student’s t-test). (c) Quantification of γH2AX, RPA and Rad51 foci at 30h versus 42h after nuclear transfer and activation. Lines represent the median no. of foci in n = 23 (untreated, γH2AX) and 25 (α-amanitin, γH2AX),n = 56 (untreated, RPA), 69 (α-amanitin, RPA), 34 (untreated, Rad51) and 42 ((α-amanitin, Rad51) number of embryos. Arrowhead indicates outlier with significant DNA damage. (d) DNA replication (EdU incorporation, top panel) and nascent RNA transcription (5-ethynyluridine, EU incorporation, bottom panel) in untreated and α-amanitin treated NT embryos. Arrowheads point to EU labelled foci indicating high transcriptional activity. (e) RPA, Rad51 and γH2AX staining in untreated and α-amanitin treated NT embryos. (f–h) Quantification of γH2AX, RPA and Rad51 foci in untreated and α-amanitin treated embryos. Line represents median number of foci. n = 23 (γH2AX, untreated), 25 (γH2AX, α-amanitin treated), 56 (RPA32, untreated), 69 (RPA32, α-amanitin treated), 34 (Rad51, untreated) and 42 (Rad5, α-amanitin treated) embryos. Scale bar: 5 μm and 10 μm (inserts).
Supplementary information
Supplementary Information
Supplementary Information (PDF 1525 kb)
Rights and permissions
About this article
Cite this article
Chia, G., Agudo, J., Treff, N. et al. Genomic instability during reprogramming by nuclear transfer is DNA replication dependent. Nat Cell Biol 19, 282–291 (2017). https://doi.org/10.1038/ncb3485
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3485
This article is cited by
-
Farrerol directly activates the deubiqutinase UCHL3 to promote DNA repair and reprogramming when mediated by somatic cell nuclear transfer
Nature Communications (2023)
-
Measurements of Electrical Characteristics of Mammalian Oocytes
Measurement Techniques (2023)
-
Chromatin architecture reorganization in murine somatic cell nuclear transfer embryos
Nature Communications (2020)
-
Efficient SNP editing in haploid human pluripotent stem cells
Journal of Assisted Reproduction and Genetics (2020)
-
Somatic cell nuclear transfer in non-enucleated goldfish oocytes: understanding DNA fate during oocyte activation and first cellular division
Scientific Reports (2019)