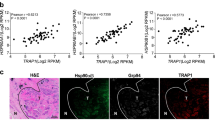
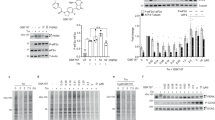
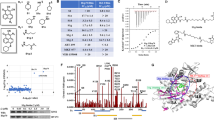
Abstract
Protein kinase clients are recruited to the Hsp90 molecular chaperone system via Cdc37, which simultaneously binds Hsp90 and kinases and regulates the Hsp90 chaperone cycle. Pharmacological inhibition of Hsp90 in vivo results in degradation of kinase clients, with a therapeutic effect in dependent tumors. We show here that Cdc37 directly antagonizes ATP binding to client kinases, suggesting a role for the Hsp90–Cdc37 complex in controlling kinase activity. Unexpectedly, we find that Cdc37 binding to protein kinases is itself antagonized by ATP-competitive kinase inhibitors, including vemurafenib and lapatinib. In cancer cells, these inhibitors deprive oncogenic kinases such as B-Raf and ErbB2 of access to the Hsp90–Cdc37 complex, leading to their degradation. Our results suggest that at least part of the efficacy of ATP-competitive inhibitors of Hsp90-dependent kinases in tumor cells may be due to targeted chaperone deprivation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pearl, L.H. & Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75, 271–294 (2006).
Pearl, L.H. Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr. Opin. Genet. Dev. 15, 55–61 (2005).
Caplan, A.J., Mandal, A.K. & Theodoraki, M.A. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 17, 87–92 (2007).
Karnitz, L.M. & Felts, S.J. Cdc37 regulation of the kinome: when to hold 'em and when to fold 'em. Sci. STKE 2007, pe22 (2007).
Roe, S.M. et al. The mechanism of Hsp90 regulation by the protein kinase–specific cochaperone p50(cdc37). Cell 116, 87–98 (2004).
Vaughan, C.K. et al. Structure of an Hsp90–Cdc37–Cdk4 complex. Mol. Cell 23, 697–707 (2006).
Mimnaugh, E.G., Chavany, C. & Neckers, L. Polyubiquitination and proteasomal degradation of the p185c–erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem. 271, 22796–22801 (1996).
Schneider, C. et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc. Natl. Acad. Sci. USA 93, 14536–14541 (1996).
Powers, M.V. & Workman, P. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett. 581, 3758–3769 (2007).
Pearl, L.H., Prodromou, C. & Workman, P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem. J. 410, 439–453 (2008).
Makhnevych, T. & Houry, W.A. The role of Hsp90 in protein complex assembly. Biochim. Biophys. Acta 1823, 674–682 (2012).
Hunter, T. & Poon, R.Y.C. Cdc37: A protein kinase chaperone? Trends Cell Biol. 7, 157–161 (1997).
Terasawa, K. & Minami, Y. A client-binding site of Cdc37. FEBS J. 272, 4684–4690 (2005).
Zhao, Q., Boschelli, F., Caplan, A.J. & Arndt, K.T. Identification of a conserved sequence motif that promotes Cdc37 and cyclin D1 binding to Cdk4. J. Biol. Chem. 279, 12560–12564 (2004).
Prince, T. & Matts, R.L. Definition of protein kinase sequence motifs that trigger high affinity binding of Hsp90 and Cdc37. J. Biol. Chem. 279, 39975–39981 (2004).
Shao, J., Irwin, A., Hartson, S.D. & Matts, R.L. Functional dissection of cdc37: characterization of domain structure and amino acid residues critical for protein kinase binding. Biochemistry 42, 12577–12588 (2003).
Prince, T. & Matts, R.L. Exposure of protein kinase motifs that trigger binding of Hsp90 and Cdc37. Biochem. Biophys. Res. Commun. 338, 1447–1454 (2005).
Xu, W. et al. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat. Struct. Mol. Biol. 12, 120–126 (2005).
da Rocha Dias, S. et al. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 65, 10686–10691 (2005).
Grbovic, O.M. et al. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc. Natl. Acad. Sci. USA 103, 57–62 (2006).
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl. Acad. Sci. USA 105, 3041–3046 (2008).
Sharp, S.Y. et al. Inhibition of the heat shock protein 90 molecular chaperone in vitro and in vivo by novel, synthetic, potent resorcinylic pyrazole/isoxazole amide analogues. Mol. Cancer Ther. 6, 1198–1211 (2007).
Johnson, L.N., Noble, M.E.M. & Owen, D.J. Active and inactive protein kinases: structural basis for regulation. Cell 85, 149–158 (1996).
Johnson, L.N. Protein kinase inhibitors: contributions from structure to clinical compounds. Q. Rev. Biophys. 42, 1–40 (2009).
Terasawa, K. et al. Cdc37 interacts with the glycine-rich loop of Hsp90 client kinases. Mol. Cell Biol. 26, 3378–3389 (2006).
Polier, S., Dragovic, Z., Hartl, F.U. & Bracher, A. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 133, 1068–1079 (2008).
Ni, Q., Shaffer, J. & Adams, J.A. Insights into nucleotide binding in protein kinase A using fluorescent adenosine derivatives. Protein Sci. 9, 1818–1827 (2000).
Hatzivassiliou, G. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464, 431–435 (2010).
Joseph, E.W. et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF–selective manner. Proc. Natl. Acad. Sci. USA 107, 14903–14908 (2010).
Miyata, Y. & Nishida, E. CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol. Cell Biol. 24, 4065–4074 (2004).
Zhang, B.H. & Guan, K.L. Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J. 19, 5429–5439 (2000).
Wan, P.T. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
King, A.J. et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 66, 11100–11105 (2006).
Farrell, A. & Morgan, D.O. Cdc37 promotes the stability of protein kinases Cdc28 and Cak1. Mol. Cell Biol. 20, 749–754 (2000).
Vaughan, C.K. et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol. Cell 31, 886–895 (2008).
Smith, J.R., Clarke, P.A., de Billy, E. & Workman, P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene 28, 157–169 (2009).
Xu, W. et al. Dynamic tyrosine phosphorylation modulates cycling of the HSP90–P50CDC37–AHA1 chaperone machine. Mol. Cell 47, 434–443 (2012).
Taipale, M. et al. Quantitative analysis of hsp90-client interactions reveals principles of substrate recognition. Cell 150, 987–1001 (2012).
Nakatani, H. et al. STI571 (Glivec) inhibits the interaction between c-KIT and heat shock protein 90 of the gastrointestinal stromal tumor cell line, GIST-T1. Cancer Sci. 96, 116–119 (2005).
Citri, A. et al. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 21, 2407–2417 (2002).
Das Thakur, M. et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 494, 251–255 (2013).
Flaherty, K.T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010).
Smith, J.R. The role of the HSP90 cochaperone CDC37 and its therapeutic potential in cancer.. PhD thesis, Univ. London, (2008).
Meier, C. et al. Engineering human MEK-1 for structural studies: a case study of combinatorial domain hunting. J. Struct. Biol. 177, 329–334 (2012).
Vaughan, C.K., Piper, P.W., Pearl, L.H. & Prodromou, C. A common conformationally coupled ATPase mechanism for yeast and human cytoplasmic HSP90s. FEBS J. 276, 199–209 (2009).
Siligardi, G. et al. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277, 20151–20159 (2002).
Holford, J., Sharp, S.Y., Murrer, B.A., Abrams, M. & Kelland, L.R. In vitro circumvention of cisplatin resistance by the novel sterically hindered platinum complex AMD473. Br. J. Cancer 77, 366–373 (1998).
Acknowledgements
We are grateful to L. Zhou (University of Sussex) for assistance with insect cell expression; to R.M.L. Morgan and S. Parry-Morris (both from University of Sussex) for provision of Hsp90 and Mek1 protein; S.H. Millson (University of Sheffield) for provision of Cak1p-containing pellets; J.R. Smith (The Institute of Cancer Research) for Cdc37 knockdown and overexpressing cell lines; and to A.W. Oliver and S.M. Roe (both from University of Sussex) for useful discussions. P.W., P.A.C. and R.S.S. acknowledge funding from the UK National Health Service to the National Institute for Health Research Biomedical Research Centre at The Institute of Cancer Research and Royal Marsden Hospital. L.H.P. acknowledges funding for the Genome Damage and Stability Centre from the Medical Research Council. This work was supported by Cancer Research UK Programme grant C309/A8274 (P.W.), a European Molecular Biology Organization Long-Term Fellowship (S.P.) and a Wellcome Trust Senior Investigator Award (095605/Z/11/Z to L.H.P.). P.W. is a Cancer Research UK Life Fellow.
Author information
Authors and Affiliations
Contributions
S.P. designed the study, performed the in vitro experiments, analyzed the data, prepared the figures and coauthored the manuscript. R.S.S. and P.A.C. performed the in vivo experiments and analyzed the data. P.W. designed the study, analyzed the data and coauthored the manuscript. C.P. designed the study, performed in vitro experiments and analyzed the data. L.H.P. designed the study, analyzed the data, prepared the figures and coauthored the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 18042 kb)
Rights and permissions
About this article
Cite this article
Polier, S., Samant, R., Clarke, P. et al. ATP-competitive inhibitors block protein kinase recruitment to the Hsp90-Cdc37 system. Nat Chem Biol 9, 307–312 (2013). https://doi.org/10.1038/nchembio.1212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1212
This article is cited by
-
HSP90-CDC37-PP5 forms a structural platform for kinase dephosphorylation
Nature Communications (2022)
-
Combined PARP and HSP90 inhibition: preclinical and Phase 1 evaluation in patients with advanced solid tumours
British Journal of Cancer (2022)
-
HSP-90/kinase complexes are stabilized by the large PPIase FKB-6
Scientific Reports (2021)
-
Glucocorticoid receptor complexes form cooperatively with the Hsp90 co-chaperones Pp5 and FKBPs
Scientific Reports (2020)
-
Unifying principles of bifunctional, proximity-inducing small molecules
Nature Chemical Biology (2020)