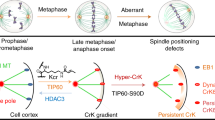
Abstract
Faithful segregation of chromosomes in mammalian cells requires bi-orientation of sister chromatids, which relies on the sensing of correct attachments between spindle microtubules and kinetochores. Although the mechanisms underlying cyclin-dependent kinase 1 (CDK1) activation, which triggers mitotic entry, have been extensively studied, the regulatory mechanisms that couple CDK1–cyclin B activity to chromosome stability are not well understood. Here, we identified a signaling axis in which Aurora B activity is modulated by CDK1–cyclin B via the acetyltransferase TIP60 in human cell division. CDK1–cyclin B phosphorylates Ser90 of TIP60, which elicits TIP60-dependent acetylation of Aurora B and promotes accurate chromosome segregation in mitosis. Mechanistically, TIP60 acetylation of Aurora B at Lys215 protects Aurora B's activation loop from dephosphorylation by the phosphatase PP2A to ensure a robust, error-free metaphase-anaphase transition. These findings delineate a conserved signaling cascade that integrates protein phosphorylation and acetylation with cell cycle progression for maintenance of genomic stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cleveland, D.W., Mao, Y. & Sullivan, K.F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407–421 (2003).
Kabeche, L. & Compton, D.A. Cyclin A regulates kinetochore microtubules to promote faithful chromosome segregation. Nature 502, 110–113 (2013).
Orthwein, A. et al. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science 344, 189–193 (2014).
Jackson, S.P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Lee, J.H. & Paull, T.T. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304, 93–96 (2004).
Sun, Y. et al. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat. Cell Biol. 11, 1376–1382 (2009).
Zachos, G. et al. Chk1 is required for spindle checkpoint function. Dev. Cell 12, 247–260 (2007).
Petsalaki, E. & Zachos, G. Chk2 prevents mitotic exit when the majority of kinetochores are unattached. J. Cell Biol. 205, 339–356 (2014).
Stolz, A. et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat. Cell Biol. 12, 492–499 (2010).
Cheng, Z. et al. Functional characterization of TIP60 sumoylation in UV-irradiated DNA damage response. Oncogene 27, 931–941 (2008).
Coffey, K. et al. Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS One 7, e45539 (2012).
Ghizzoni, M. et al. 6-alkylsalicylates are selective Tip60 inhibitors and target the acetyl-CoA binding site. Eur. J. Med. Chem. 47, 337–344 (2012).
Dou, Z. et al. Dynamic localization of Mps1 to kinetochore is essential for accurate spindle microtubule attachment. Proc. Natl. Acad. Sci. USA 112, E4546–E4555 (2015).
Ji, Z., Gao, H. & Yu, H. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 348, 1260–1264 (2015).
Hiruma, Y. et al. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 348, 1264–1267 (2015).
Martin-Lluesma, S., Stucke, V.M. & Nigg, E.A. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270 (2002).
Wood, K.W. et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proc. Natl. Acad. Sci. USA 107, 5839–5844 (2010).
Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W.C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803 (2012).
Lampson, M.A., Renduchitala, K., Khodjakov, A. & Kapoor, T.M. Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 6, 232–237 (2004).
Posch, M. et al. Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis. J. Cell Biol. 191, 61–74 (2010).
Zeitlin, S.G., Shelby, R.D. & Sullivan, K.F. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155, 1147–1157 (2001).
Yasui, Y. et al. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J. Biol. Chem. 279, 12997–13003 (2004).
Sugiyama, K. et al. Aurora-B associated protein phosphatases as negative regulators of kinase activation. Oncogene 21, 3103–3111 (2002).
Cohen, P., Klumpp, S. & Schelling, D.L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 250, 596–600 (1989).
Favre, B., Turowski, P. & Hemmings, B.A. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 272, 13856–13863 (1997).
Neumann, H., Peak-Chew, S.Y. & Chin, J.W. Genetically encoding Nɛ-acetyllysine in recombinant proteins. Nat. Chem. Biol. 4, 232–234 (2008).
Neumann, H. et al. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3 K56 acetylation. Mol. Cell 36, 153–163 (2009).
Honda, R., Körner, R. & Nigg, E.A. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 14, 3325–3341 (2003).
Ruchaud, S., Carmena, M. & Earnshaw, W.C. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 8, 798–812 (2007).
Lemercier, C. et al. Tip60 acetyltransferase activity is controlled by phosphorylation. J. Biol. Chem. 278, 4713–4718 (2003).
Charvet, C. et al. Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol. Cell 42, 584–596 (2011).
Lin, S.Y. et al. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science 336, 477–481 (2012).
Skoufias, D.A., Indorato, R.L., Lacroix, F., Panopoulos, A. & Margolis, R.L. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J. Cell Biol. 179, 671–685 (2007).
Foley, E.A. & Kapoor, T.M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 14, 25–37 (2013).
Nijenhuis, W., Vallardi, G., Teixeira, A., Kops, G.J. & Saurin, A.T. Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat. Cell Biol. 16, 1257–1264 (2014).
Holland, A.J. & Cleveland, D.W. Chromoanagenesis and cancer: mechanisms and consequences of localized, complex chromosomal rearrangements. Nat. Med. 18, 1630–1638 (2012).
Kaidi, A. & Jackson, S.P. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 498, 70–74 (2013).
Nishino, T. et al. CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell 148, 487–501 (2012).
Tao, Y. et al. The structure of the FANCM-MHF complex reveals physical features for functional assembly. Nat. Commun. 3, 782 (2012).
Janssen, A., van der Burg, M., Szuhai, K., Kops, G.J. & Medema, R.H. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333, 1895–1898 (2011).
Xia, P. et al. EB1 acetylation by P300/CBP-associated factor (PCAF) ensures accurate kinetochore-microtubule interactions in mitosis. Proc. Natl. Acad. Sci. USA 109, 16564–16569 (2012).
Xiao, Y. et al. Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Reports 7, 1471–1480 (2014).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Dou, Z. et al. TTK kinase is essential for the centrosomal localization of TACC2. FEBS Lett. 572, 51–56 (2004).
Liu, D. et al. Human NUF2 interacts with centromere-associated protein E and is essential for a stable spindle microtubule-kinetochore attachment. J. Biol. Chem. 282, 21415–21424 (2007).
Chu, Y. et al. Aurora B kinase activation requires survivin priming phosphorylation by PLK1. J. Mol. Cell Biol. 3, 260–267 (2011).
Yang, Y. et al. Phosphorylation of HsMis13 by Aurora B kinase is essential for assembly of functional kinetochore. J. Biol. Chem. 283, 26726–26736 (2008).
Yao, X., Anderson, K.L. & Cleveland, D.W. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J. Cell Biol. 139, 435–447 (1997).
Ding, X. et al. Probing CENP-E function in chromosome dynamics using small molecule inhibitor syntelin. Cell Res. 20, 1386–1389 (2010).
Acknowledgements
We are grateful to Y. Shi (University of Science & Technology of China) and Y. Chen (Natural Science Foundation of China) for support; to S. Zhao (Fudan University) for mass spectrometric assistance; and to J. Chin (MRC Laboratory of Molecular Biology, Cambridge, UK) for reagents. This work was supported in part by the Natural Science Foundation of China (grants 31430054, 31320103904 and 91313303, 2002CB713700 to X.Y.; 31501095 to X.L.; 81270466 to X.D.; 31371363 to Z.D.; 31271439 to C.F.; and 91213303 to Z.W.), 973 projects (2014CB964803 to X.Y.; 2012CB917200 to J.Za., L.N.; 2012CB945002, 2013CB911203 to Z.D.; 2002CB713701 to C.F.); MOE Innovative team IRT13038, Fundamental Research Funds for the Central Universities WK2070000066; Chinese Academy of Sciences Center of Excellence 2015HSC-UE010; and the US National Institutes of Health (DK56292 and CA164133 to X.Y.).
Author information
Authors and Affiliations
Contributions
X.Y. and G.F. conceived the project. F.M., X.Z. and X.L. designed and performed most biochemical and cell biological experiments. P.Y.Y., B.Q., Z.S., J.Za., Z.W. and J.Zh. performed chemical biological experiments and evaluated small molecule inhibitors. Z.D., C.T., M.T., L.N. and C.F. assisted in recombinant protein engineering and purification. F.M., X.Z., X.L., P.Y.Y., B.Q., Z.S., J.Za., C.F. and X.D. performed data analyses. F.M., X.Z., X.L. and X.Y. wrote the manuscript. D.L.H. and G.F. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–9. (PDF 9371 kb)
Rights and permissions
About this article
Cite this article
Mo, F., Zhuang, X., Liu, X. et al. Acetylation of Aurora B by TIP60 ensures accurate chromosomal segregation. Nat Chem Biol 12, 226–232 (2016). https://doi.org/10.1038/nchembio.2017
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2017
This article is cited by
-
Phase separation of EB1 guides microtubule plus-end dynamics
Nature Cell Biology (2023)
-
Unconventional roles of chromatin remodelers and long non-coding RNAs in cell division
Cellular and Molecular Life Sciences (2023)
-
Evolutionary conserved relocation of chromatin remodeling complexes to the mitotic apparatus
BMC Biology (2022)
-
Modulation of cellular processes by histone and non-histone protein acetylation
Nature Reviews Molecular Cell Biology (2022)
-
Loss of TIP60 (KAT5) abolishes H2AZ lysine 7 acetylation and causes p53, INK4A, and ARF-independent cell cycle arrest
Cell Death & Disease (2022)