Abstract
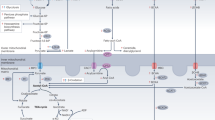
Despite advances in treatment, chronic heart failure is still associated with significant morbidity and a poor prognosis. The scope for further advances based on additional neurohumoral blockade is small. Effective adjunctive therapies acting via a different cellular mechanism would, therefore, be attractive. Energetic impairment seems to contribute to the pathogenesis of heart failure. The findings from several studies have shown that the so-called metabolic agents could have potential as adjunctive therapies in heart failure. These agents cause a shift in the substrate used by the heart away from free fatty acids, the oxidation of which normally provides around 70% of the energy needed, towards glucose. The oxygen cost of energy generation is lessened when glucose is used as the substrate. In this review we aim to draw attention to the metabolic alteration in heart failure and we present evidence supporting the use of metabolic therapy in heart failure.
Key Points
-
Emerging evidence shows that, irrespective of the etiology, an energetic impairment contributes to the pathogenesis of heart failure
-
Patterns of substrate use in heart failure are complex and depend on species, cause, duration, underlying coronary artery disease, endothelial dysfunction, and the presence of genetic and other comorbidities
-
Myocardial use of fatty acids costs more oxygen per unit of ATP generated than glucose
-
Animal models and small-scale human studies suggest benefits with the use of agents that shift myocardial substrate use from free fatty acids towards glucose, but larger human studies are needed
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Unverferth DV et al. (1988) Human myocardial adenosine triphosphatase activities in health and heart failure. Am Heart J 115: 139–146
Starling RC et al. (1998) Human myocardial ATP content and in vivo contractile function. Mol Cell Biochem 180: 171–177
Beer M et al. (2002) Absolute concentrations of high-energy phosphate metabolites in normal hypertrophied and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40: 1267–1274
Shen W et al. (1999) Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs: a compensatory role for the parallel loss of creatine. Circulation 100: 2113–2118
Bittl JA and Ingwall JS (1985) Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem 260: 3512–3517
Weiss RG et al. (2005) ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA 102: 808–813
Tian R and Ingwall JS (1999) The molecular energetics of the failing heart from animal models—small animal models. Heart Failure Rev 4: 245–253
Zhang J and Bache R (1999) The molecular energetics of the failing heart from animal models—large animal models. Heart Failure Rev 4: 255–267
Nakae I et al. (2003) Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol 42: 1587–1593
De Sousa E et al. (1999) Subcellular creatine kinase alterations. Implications in heart failure. Circ Res 85: 68–76
Conway MA et al. (1991) Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338: 973–976
Neubauer S et al. (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96: 2190–2196
Gross WL et al. (1996) Nitric oxide inhibits creatine kinase and regulates rat heart contractile reserve. Proc Natl Acad Sci USA 93: 5604–5609
Andrews R et al. (1998) The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J 19: 617–622
Arany Z et al. (2005) Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271
Sack MN et al. (2000) Coordinate regulation of metabolic enzyme encoding genes during cardiac development and following carvedilol therapy in spontaneously hypertensive rats. Cardiovasc Drugs Ther 14: 31–39
Stanley WC et al. (1997) Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res 33: 243–257
Stanley WC et al. (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129
Kagaya Y et al. (1990) Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. A quantitative autoradiographic study. Circulation 81: 1353–1361
Alaoui-Talibi Z et al. (1997) Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-L-carnitine. Am J Physiol 272: H1615–H1624
Nikolaidis LA et al. (2004) The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res 61: 297–306
Lommi J et al. (1998) Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol 81: 45–50
Taylor M et al. (2001) An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in patients with congestive heart failure. J Nucl Med 42: 55–62
Randle PJ (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14: 263–283
Mansson S et al. (2005) 13C imaging—a new diagnostic platform. Eur Radiol 16: 57–67
Myrmel T et al. (1992) Triacylglycerol metabolism in hypoxic, glucose-deprived rat cardiomyocytes. J Mol Cell Cardiol 24: 855–868
Korvald C et al. (2000) Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol 278: H1345–H1351
Boehm EA et al. (2001) Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am J Physiol Heart Circ Physiol 280: H977–H983
Schrauwen P et al. (2003) Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB J 17: 2272–2274
Murray AJ et al. (2004) Uncoupling proteins in human heart. Lancet 364: 1786–1788
Kjekshus JK and Mjos OD (1972) Effect of free fatty acids on myocardial function and metabolism in the ischemic dog heart. J Clin Invest 51: 1767–1776
Hermann HP et al. (1999) Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet 353: 1321–1323
Kantor PF et al. (2000) The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 86: 580–588
MacInnes A et al. (2003) The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 93: e26–e32
Szwed H et al. (2001) Combination treatment in stable effort angina using trimetazidine and metoprolol: results of a randomized, double-blind, multicentre study (TRIMPOL II). TRIMetazidine in POLand. Eur Heart J 22: 2267–2274
Vitale C et al. (2004) Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J 25: 1814–1821
Di Napoli P et al. (2005) Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart 91: 161–165
Rosano GM et al. (2003) Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol 2: 16
Thrainsdottir IS et al. (2004) Effects of trimetazidine on left ventricular function in patients with type 2 diabetes and heart failure. J Cardiovasc Pharmacol 44: 101–108
McCormack JG et al. (1996) Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation 93: 135–142
Clarke B et al. (1996) Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol 28: 341–350
McCullough PA (2005) Chronic angina: new medical options for treatment. Rev Cardiovasc Med 6: 152–161
Chandler MP et al. (2002) Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res 91: 278–280
Sabbah HN et al. (2002) Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail 8: 416–422
Leotta G et al. (2005) Relationship between QT interval and cardiovascular risk factors in healthy young subjects. J Hum Hypertens 19: 623–627
Lionetti V et al. (2005) Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res 66: 454–461
Greaves P et al. (1984) Cardiac hypertrophy in the dog and rat induced by oxfenicine, an agent which modifies muscle metabolism. Arch Toxicol Suppl 7: S488–S493
Bachmann E and Weber E (1988) Biochemical mechanisms of oxfenicine cardiotoxicity. Pharmacology 36: 238–248
Abdel-aleem S et al. (1994) Regulation of glucose utilization during the inhibition of fatty acid oxidation in rat myocytes. Horm Metab Res 26: 88–91
Reaven GM et al. (1988) Additive hypoglycemic effects of drugs that modify free-fatty acid metabolism by different mechanisms in rats with streptozocin-induced diabetes. Diabetes 37: 28–32
Turcani M and Rupp H (1999) Modification of left ventricular hypertrophy by chronic etomixir treatment. Br J Pharmacol 126: 501–507
Lopaschuk GD and Spafford M (1989) Response of isolated working hearts to fatty acids and carnitine palmitoyltransferase I inhibition during reduction of coronary flow in acutely and chronically diabetic rats. Circ Res 65: 378–387
Schmidt-Schweda S and Holubarsch C (2000) First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 99: 27–35
Bristow MR et al. (1996) Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 94: 2807–2816
Packer M et al. (1996) Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation 94: 2793–2799
Wallhaus TR et al. (2001) Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 103: 2441–2446
Al Hesayen A et al. (2005) Selective versus nonselective β-adrenergic receptor blockade in chronic heart failure: differential effects on myocardial energy substrate utilization. Eur J Heart Fail 7: 618–623
Jeffrey FM et al. (1995) Direct evidence that perhexiline modifies myocardial substrate utilization from fatty acids to lactate. J Cardiovasc Pharmacol 25: 469–472
Kennedy JA et al. (1996) Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharmacol 52: 273–280
Kennedy JA et al. (2000) Effect of perhexiline and oxfenicine on myocardial function and metabolism during low-flow ischemia/reperfusion in the isolated rat heart. J Cardiovasc Pharmacol 36: 794–801
Horowitz JD and Mashford ML (1979) Perhexiline maleate in the treatment of severe angina pectoris. Med J Aust 1: 485–488
Cole PL et al. (1990) Efficacy and safety of perhexiline maleate in refractory angina. A double-blind placebo-controlled clinical trial of a novel antianginal agent. Circulation 81: 1260–1270
Pessayre D et al. (1979) Perhexiline maleate-induced cirrhosis. Gastroenterology 76: 170–177
Bouche P et al. (1979) Perhexiline maleate and peripheral neuropathy. Neurology 29: 739–743
Morgan MY et al. (1984) Impaired oxidation of debrisoquine in patients with perhexiline liver injury. Gut 25: 1057–1064
Horowitz JD et al. (1986) Perhexiline maleate treatment for severe angina pectoris—correlations with pharmacokinetics. Int J Cardiol 13: 219–229
Klassen GA et al. (1976) Effects of perhexiline maleate on coronary flow distribution in the ischemic canine myocardium. Circulation 54: 14–20
Lee L et al. (2005) Metabolic modulation with perhexiline in chronic heart failure: a randomized controlled trial of short-term use of a novel treatment. Circulation 112: 3280–3288
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Michael Frenneaux has applied for a patent for the use of perhexiline therapy in chronic heart failure patients. The other authors declared they have no competing interests.
Rights and permissions
About this article
Cite this article
Abozguia, K., Clarke, K., Lee, L. et al. Modification of myocardial substrate use as a therapy for heart failure. Nat Rev Cardiol 3, 490–498 (2006). https://doi.org/10.1038/ncpcardio0583
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0583
This article is cited by
-
Inhibition of Lipid Oxidation Increases Glucose Metabolism and Enhances 2-Deoxy-2-[18F]Fluoro-d-Glucose Uptake in Prostate Cancer Mouse Xenografts
Molecular Imaging and Biology (2015)
-
Mesenchymal stem cell transplantation for the infarcted heart: therapeutic potential for insulin resistance beyond the heart
Cardiovascular Diabetology (2013)
-
Ranolazine: Clinical Applications and Therapeutic Basis
American Journal of Cardiovascular Drugs (2013)
-
Early diastolic function during exertion influences exercise intolerance in patients with hypertrophic cardiomyopathy
Journal of Echocardiography (2013)
-
Angiotensin-converting enzyme inhibition and food restriction in diabetic mice do not correct the increased sensitivity for ischemia-reperfusion injury
Cardiovascular Diabetology (2012)