Abstract
Thyroid hormones influence gene expression in virtually all vertebrate tissues. Precise regulation of the active endogenous ligand, 3,5,3'-triiodothyronine (T3), is achieved by the sequential removal of iodine moieties from the thyroid hormone molecule. Type III iodothyronine deiodinase (D3) is the major inactivating enzyme terminating the action of T3 and preventing activation of the prohormone, thyroxine (T4). Recent studies have revealed the induction of high D3 activity in diverse animal models of tissue injury including starvation, cryolesion, cardiac hypertrophy, infarction, and chronic inflammation. By analyzing serum and tissues taken from hospitalized patients at the time of death, investigators have also documented the robust induction of D3 activity in several human tissues that normally have none, including the liver and skeletal muscle, and shown clinically relevant consequences to systemic thyroid status. These studies reveal a novel role of D3 in the tissue response to injury and in the derangement of thyroid hormone homeostasis commonly observed during critical illness.
Key Points
-
The low-T3 syndrome is multifactorial, with evidence that decreased glandular secretion, decreased T4 activation, and increased thyroid hormone inactivation all contribute
-
Type III iodothyronine deiodinase (D3) is normally undetectable in mature tissues, but its expression is re-activated in diverse cell types in response to injury
-
D3 re-activation during illness is associated with a fall in serum T3 levels
-
Additional studies are required to identify the molecular mechanisms that regulate the re-activation of D3 during illness and the functional consequence of D3-mediated local hypothyroidism to injured tissues
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Brent GA (1994) The molecular basis of thyroid hormone action. N Engl J Med 331: 847–853
Flamant F et al. (2007) Thyroid hormones signaling is getting more complex: STORMs are coming. Mol Endocrinol 21: 321–333
Bianco AC and Kim BW (2006) Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116: 2571–2579
Bianco AC et al. (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23: 38–89
Hernandez A (2005) Structure and function of the type 3 deiodinase gene. Thyroid 15: 865–874
Huang SA (2005) Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid 15: 875–881
Koenig RJ (2005) Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid 15: 835–840
Adler SM and Wartofsky L (2007) The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 36: 657–672, vi
De Groot LJ (1999) Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab 84: 151–164
Rodriguez-Perez A et al. (2007) Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf) [doi: 10.1111/j.1365-2265.2007.03102.x]
Peeters RP et al. (2003) Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 88: 3202–3211
Peeters RP et al. (2005) Serum 3,3',5'-triiodothyronine (rT3) and 3,5,3'-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with post-mortem tissue deiodinase activities. J Clin Endocrinol Metab 90: 4559–4565
Van der Geyten S et al. (1999) Regulation of thyroid hormone metabolism during fasting and refeeding in chicken. Gen Comp Endocrinol 116: 272–280
Li WW et al. (2001) Induction of type 3 iodothyronine deiodinase by nerve injury in the rat peripheral nervous system. Endocrinology 142: 5190–5197
Wassen FW et al. (2002) Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology 143: 2812–2815
Olivares EL et al. (2007) Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology 148: 4786–4792
Boelen A et al. (2005) Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinology 146: 5128–5134
Dentice M et al. (2007) Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci U S A 104: 14466–14471
Salvatore D et al. (1995) Type 3 lodothyronine deiodinase: cloning, in vitro expression, and functional analysis of the placental selenoenzyme. J Clin Invest 96: 2421–2430
Hernandez A et al. (1998) Localization of the type 3 iodothyronine deiodinase (DIO3) gene to human chromosome 14q32 and mouse chromosome 12F1. Genomics 53: 119–121
Hernandez A et al. (2002) The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology 143: 4483–4486
Seitz H et al. (2003) Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat Genet 34: 261–262
Davis E et al. (2005) RNAi-mediated allelic trans-interaction at the imprinted Rtl1/Peg11 locus. Curr Biol 15: 743–749
Callebaut I et al. (2003) The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J Biol Chem 278: 36887–36896
Curcio-Morelli C et al. (2003) In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology 144: 937–946
Baqui M et al. (2003) Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem 278: 1206–1211
Friesema EC et al. (2006) Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20: 2761–2772
Mortimer RH et al. (1996) Maternal to fetal thyroxine transmission in the human term placenta is limited by inner ring deiodination. J Clin Endocrinol Metab 81: 2247–2249
Huang H et al. (1999) Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that overexpress type III deiodinase. Proc Natl Acad Sci U S A 96: 962–967
Marsh-Armstrong N et al. (1999) Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron 24: 871–878
Hernandez A et al. (2006) Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116: 476–484
Hernandez A et al. (2007) Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 148: 5680–5687
Chopra IJ (1976) An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest 58: 32–40
Hennemann G (1986) Thyroid hormone deiodination in healthy man. In Thyroid Hormone Metabolism, 277–295 (Ed. Hennemann G) New York: Marcel Dekker
Campos-Barros A et al. (1996) Phenolic and tyrosyl ring iodothyronine deiodination and thyroid hormone concentrations in the human central nervous system. J Clin Endocrinol Metab 81: 2179–2185
Slominski A et al. (2002) Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol 119: 1449–1455
Santini F et al. (2003) Role for inner ring deiodination preventing transcutaneous passage of thyroxine. J Clin Endocrinol Metab 88: 2825–2830
Nauman P et al. (2004) The concentration of thyroid hormones and activities of iodothyronine deiodinases are altered in human brain gliomas. Folia Neuropathol 42: 67–73
Ruppe MD et al. (2005) Consumptive hypothyroidism caused by paraneoplastic production of type 3 iodothyronine deiodinase. Thyroid 15: 1369–1372
Huang SA et al. (2000) Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. New Engl J Med 343: 185–189
Huang SA et al. (2002) A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab 87: 4457–4461
Bianco AC et al. (1987) The role of glucocorticoids in the stress-induced reduction of extrathyroidal 3,5,3'-triiodothyronine generation in rats. Endocrinology 120: 1033–1038
Chopra IJ et al. (1987) Evidence against benefit from replacement doses of thyroid hormones in nonthyroidal illness (NTI): studies using turpentine oil-injected rat. J Endocrinol Invest 10: 559–564
Gartner W and Weissel M (2004) Do iodine-containing contrast media induce clinically relevant changes in thyroid function parameters of euthyroid patients within the first week. Thyroid 14: 521–524
Brown RS et al. (1997) Routine skin cleansing with povidone-iodine is not a common cause of transient neonatal hypothyroidism in North America: a prospective controlled study. Thyroid 7: 395–400
Nagaya T et al. (2000) A potential role of activated NF-κB in the pathogenesis of euthyroid sick syndrome. J Clin Invest 106: 393–402
Yu J and Koenig RJ (2000) Regulation of hepatocyte thyroxine 5'-deiodinase by T3 and nuclear receptor coactivators as a model of the sick euthyroid syndrome. J Biol Chem 275: 38296–38301
Yu J and Koenig RJ (2006) Induction of type 1 iodothyronine deiodinase to prevent the nonthyroidal illness syndrome in mice. Endocrinology 147: 3580–3585
Mebis L et al. (2007) The type II iodothyronine deiodinase is up-regulated in skeletal muscle during prolonged critical illness. J Clin Endocrinol Metab 92: 3330–3333
Saberi M et al. (1975) Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. J Clin Invest 55: 218–223
Nicoloff JT (1970) A new method for the measurement of acute alterations in thyroxine deiodination rate in man. J Clin Invest 49: 267–273
Luiza Maia A et al. (2005) Type 2 iodothyronine deiodinase is the major source of plasma T(3) in euthyroid humans. J Clin Invest 115: 2524–2533
St Germain DL et al. (2005) Insights into the role of deiodinases from studies of genetically modified animals. Thyroid 15: 905–916
Christoffolete MA et al. (2007) Mice with impaired extrathyroidal thyroxine to 3,5,3'-triiodothyronine conversion maintain normal serum 3,5,3'-triiodothyronine concentrations. Endocrinology 148: 954–960
Brent GA and Hershman JM (1986) Thyroxine therapy in patients with severe nonthyroidal illness and low serum thyroxine concentrations. J Clin Endocrinol Metab 63: 1–8
Hernandez A and Obregon MJ (1995) Presence of growth factors-induced type III iodothyronine 5-deiodinase in cultured rat brown adipocytes. Endocrinology 136: 4543–4550
Pallud S et al. (1999) Regulation of type 3 iodothyronine deiodinase expression in cultured rat astrocytes: role of the Erk cascade. Endocrinology 140: 2917–2923
Kester MH et al. (2006) Regulation of type III iodothyronine deiodinase expression in human cell lines. Endocrinology 147: 5845–5854
Huang SA et al. (2005) Transforming growth factor-β promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol Endocrinol 19: 3126–3136
Bianco AC and Silva JE (1988) Cold exposure rapidly induces virtual saturation of brown adipose tissue nuclear T3 receptors. Am J Physiol 255: E496–E503
de Jesus LA et al. (2001) The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108: 1379–1385
Buermans HP et al. (2005) Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 21: 314–323
Baqui MM et al. (2000) Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology 141: 4309–4312
Dentice M et al. (2005) The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol 7: 698–705
Sagar GD et al. (2007) Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol 27: 4774–4783
Silva JE and Larsen PR (1977) Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science 198: 617–620
Silva JE et al. (1982) Evidence for two tissue specific pathways for in vivo thyroxine 5' deiodination in the rat. J Clin Invest 69: 1176–1184
Acknowledgements
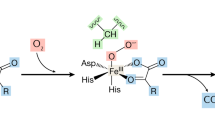
The authors are supported by grants DK58538, DK65055, DK77148, and DK76099 from the National Institutes of Health, and the Doris Duke Clinical Scientist Development Award from the Doris Duke Charitable Foundation. We thank Scott Ribich for his helpful comments and the design of Figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Huang, S., Bianco, A. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Rev Endocrinol 4, 148–155 (2008). https://doi.org/10.1038/ncpendmet0727
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0727
This article is cited by
-
Thyroid function in the subacute phase of traumatic brain injury: a potential predictor of post-traumatic neurological and functional outcomes
Journal of Endocrinological Investigation (2022)
-
Low triiodothyronine levels correlate with high B-type natriuretic peptide levels in patients with heart failure
Scientific Reports (2021)
-
Advances in TRH signaling
Reviews in Endocrine and Metabolic Disorders (2016)