Abstract
Sodium channels can provide a route for a persistent influx of sodium ions into neurons. Over the past decade, it has emerged that sustained sodium influx can, in turn, trigger calcium ion influx, which produces axonal injury in neuroinflammatory disorders such as multiple sclerosis (MS). The development of sodium channel blockers as potential neuroprotectants in MS has proceeded rapidly, and two clinical trials are currently ongoing. The route from the laboratory to the clinic includes some complex turns, however, and a third trial was recently put on hold because of new data that suggested that sodium channel blockers might have multiple, complex actions. This article reviews the development of the concept of sodium channel blockers as neuroprotectants in MS, the path from laboratory to clinic, and the current status of research in this area.
Key Points
-
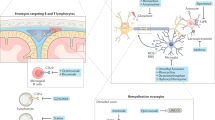
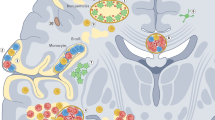
Voltage-gated sodium channels can contribute to axonal injury in multiple sclerosis (MS) by providing a pathway for sustained sodium influx that drives the Na+/Ca2+ exchanger to import calcium into axons
-
Sodium channel blockers protect axons from degeneration in several in vitro models of axonal injury, and they prevent axon degeneration, maintain impulse conduction, and improve clinical status in experimental autoimmune encephalomyelitis, a mouse model of MS
-
Sodium channels regulate the function of macrophages and microglia, so, in addition to a direct protective effect on axons, sodium channel blockers might have an immunomodulatory action
-
Sudden withdrawal of the sodium channel blockers phenytoin and carbamazepine from mice with experimental autoimmune encephalomyelitis results in acute clinical exacerbation, accompanied by increased inflammatory infiltrate within the CNS
-
Until more is known about the effects of sodium channel blocker withdrawal in humans with MS, clinical studies should monitor patients closely both in terms of neurological function and axonal loss and with respect to immune and inflammatory status
-
If withdrawal of the sodium channel blocker is necessary in patients with MS treated with carbamazepine or phenytoin for trigeminal neuralgia or other positive disturbances, these medications should be discontinued via a gradual taper
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Espir MLE and Millac P (1970) Treatment of paroxysmal disorder in multiple sclerosis with carbamazepine (Tegretol). J Neurol Neurosurg Psychiatry 33: 528–531
Hooge JP and Redekop WK (1995) Trigeminal neuralgia in multiple sclerosis. Neurology 45: 1294–1296
Sakurai M and Kanazawa I (1999) Positive symptoms in multiple sclerosis: their treatment with sodium channel blockers, lidocaine and mexiletine. J Neurol Sci 162: 162–168
Kapoor R et al. (1997) Slow sodium-dependent potential oscillations contribute to ectopic firing in mammalian demyelinated axons. Brain 120: 647–652
Schauf CL and Davis FA (1974) Impulse conduction in multiple sclerosis: a theoretical basis for modification by temperature and pharmacological agents. J Neurol Neurosurg Psychiatry 37: 152–161
Ramsaransing G et al. (2000) Worsening of symptoms of multiple sclerosis associated with carbamazepine. BMJ 320: 1113
Sakurai M et al. (1992) Lidocaine unmasks silent demyelinative lesions in multiple sclerosis. Neurology 42: 2088–2093
Solaro C et al. (2005) Antiepileptic medications in multiple sclerosis: adverse effects in a three-year follow-up study. Neurol Sci 25: 307–310
Stys PK et al. (1992) Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na+–Ca2+ exchanger. J Neurosci 12: 430–439
Herzog RI et al. (2003) Calmodulin binds to the C-terminus of sodium channels Nav 1.4 and Nav 1.6 and differentially modulates their functional properties. J Neurosci 23: 8261–8270
Nikolaeva MA et al. (2005) Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci 25: 9960–9967
Stys PK and Jiang Q (2002) Calpain-dependent neurofilament breakdown in anoxic and ischemic rat central axons. Neurosci Lett 328: 150–154
Waxman SG (1998) Demyelinating diseases: new pathological insights, new therapeutic targets. N Engl J Med 338: 323–325
Craner MJ et al. (2004) Molecular changes in neurons in MS: altered axonal expression of Nav 1.2 and Nav 1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci USA 101: 8168–8173
Prineas JW and Connell F (1979) Remyelination in multiple sclerosis. Ann Neurol 5: 22–31
Patrikios P et al. (2006) Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129: 3165–3172
Smith KJ (2006) Axonal protection in multiple sclerosis—a particular need during remyelination. Brain 129: 3147–3149
Stys PK et al. (1992) Tertiary and quaternary local anesthetics protect CNS white matter from anoxic injury at concentrations that do not block excitability. J Neurophysiol 67: 236–240
Stys PK and Lesiuk H (1996) Correlation between electrophysiological effects of mexiletine and ischemic protection in central nervous system white matter. Neuroscience 71: 27–36
Fern R et al. (1993) Pharmacological protection of CNS white matter during anoxia: actions of phenytoin, carbamazepine and diazepam. J Pharmacol Exper Ther 266: 1549–1555
Stys PK (1995) Protective effects of antiarrhythmic agents against anoxic injury in CNS white matter. J Cereb Blood Flow Metab 15: 425–432
Smith KJ et al. (2001) Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol 49: 470–476
Smith KJ and Lassmann H (2002) The role of nitric oxide in multiple sclerosis. Lancet Neurol 1: 232–241
Bolanos JP et al. (1997) Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem 68: 2227–2240
Zielasek J et al. (1995) Inhibition of brain macrophage/microglial respiratory chain enzyme activity in experimental autoimmune encephalomyelitis of the Lewis rat. Neurosci Lett 184: 129–132
Kapoor R et al. (2003) Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann Neurol 53: 174–180
Garthwaite G et al. (2002) Nitric oxide toxicity in CNS white matter: an in vitro study using rat optic nerve. Neuroscience 109: 145–155
Rush AM et al. (2005) Electrophysiological properties of two axonal sodium channels, Nav 1.2 and Nav 1.6, expressed in spinal sensory neurons. J Physiol 564: 803–816
Craner MJ et al. (2004) Co-localization of sodium channel Nav 1.6 and the sodium–calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127: 294–303
Ferguson B et al. (1997) Axonal damage in acute multiple sclerosis lesions. Brain 120: 393–399
Trapp BD et al. (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338: 323–325
Filippi M et al. (2003) Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain 126: 433–437
Davie CA et al. (1995) Persistent functional deficit in multiple sclerosis and autosomal dominant cerebellar ataxia is associated with axon loss. Brain 118: 1583–1592
Losseff NA et al. (1996) Spinal cord atrophy and disability in multiple sclerosis: a new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119: 701–708
De Stefano N et al. (1998) Axonal damage correlates with disability in patients with relapsing–remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121: 1469–1477
Bjartmar C et al. (2000) Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 48: 893–901
Dutta R et al. (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59: 478–489
Lassmann H (2003) Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci 206: 187–191
Aboul-Enein F et al. (2003) Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol 62: 25–33
Stys P (2004) Axonal degeneration in multiple sclerosis: is it time for neuroprotective strategies. Ann Neurol 55: 601–603
Lo AC et al. (2002) Neuroprotection of axons with phenytoin in experimental allergic encephalomyelitis. Neuroreport 13: 1909–1912
Lo AC et al. (2003) Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J Neurophysiol 90: 3566–3572
Bechtold DA et al. (2002) Axonal protection mediated by flecainide therapy in experimental inflammatory demyelinating disease. J Neurol 249 (Suppl 1): S204
Bechtold DA et al. (2004) Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann Neurol 55: 607–616
Bechtold DA et al. (2006) Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J Neurol 253: 1542–1551
Craner MJ et al. (2005) Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 49: 220–229
Kapoor R (2006) Neuroprotection in multiple sclerosis: therapeutic strategies and clinical trial design. Curr Opin Neurol 19: 255–259
Black JA et al. (2007) Exacerbation of EAE after withdrawal of phenytoin and carbamazepine. Ann Neurol 62: 21–33
Carrithers MD et al. (2007) Expression of the voltage-gated sodium channel Nav 1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol 178: 7822–7832
Fraser SP et al. (2004) T-lymphocyte invasiveness: control by voltage-gated Na+ channel activity. FEBS Lett 569: 191–194
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Waxman, S. Mechanisms of Disease: sodium channels and neuroprotection in multiple sclerosis—current status. Nat Rev Neurol 4, 159–169 (2008). https://doi.org/10.1038/ncpneuro0735
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0735
This article is cited by
-
A review of possible therapies for multiple sclerosis
Molecular and Cellular Biochemistry (2021)
-
Cerebral Vasoreactivity as an Indirect MRI Marker of White Matter Tracts Alterations in Multiple Sclerosis
Brain Topography (2021)
-
Fenamates Inhibit Human Sodium Channel Nav1.2 and Protect Glutamate-Induced Injury in SH-SY5Y Cells
Cellular and Molecular Neurobiology (2020)
-
Nav1.6 promotes inflammation and neuronal degeneration in a mouse model of multiple sclerosis
Journal of Neuroinflammation (2019)
-
Progressive multiple sclerosis: from pathophysiology to therapeutic strategies
Nature Reviews Drug Discovery (2019)