Abstract
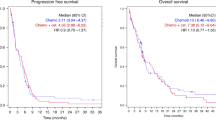
Patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) have a poor prognosis, particularly those whose disease has progressed on previous platinum-containing therapy. The epidermal growth factor receptor (EGFR) is expressed at very high levels in SCCHN and is associated with a poor prognosis. Several phase II–III studies have shown that the EGFR-targeting monoclonal antibody, cetuximab, offers clinical benefit for patients with SCCHN. Cetuximab monotherapy is active in patients whose cancer progresses on platinum-containing therapy. Tumor response and patient survival are in excess of what is achieved with commonly used therapies in this setting. Addition of a platinum regimen to cetuximab in patients with disease that progresses on platinum seems to confer no further benefit over cetuximab alone, either in terms of response rate or survival. In the first-line setting, cetuximab plus platinum and 5-fluorouracil significantly prolongs overall survival compared with platinum and 5-fluorouracil alone. The superior survival observed with cetuximab compared with platinum-based treatment demonstrates that cetuximab is the most active treatment for recurrent and/or metastatic SCCHN, and is of particular clinical significance.
Key Points
-
Cetuximab is approved for use in combination with radiotherapy in locally advanced squamous cell carcinoma of the head and neck (SCCHN) and in combination with irinotecan for the treatment of patients with EGFR-expressing, KRAS wild-type metastatic colorectal cancer
-
Administration of cetuximab at the recommended dose can be achieved without the need for modification in the dose or schedule of planned chemotherapy
-
Phase II studies of cetuximab in combination with platinum-based chemotherapy showed there was no indication that cetuximab increased the toxicity expected with platinum therapy
-
Cetuximab monotherapy offers an active approach for patients with recurrent and/or metastatic SCCHN progressing on prior platinum therapy who are unable or unwilling to undergo treatment with chemotherapy
-
Adding cetuximab to platinum/5-FU improves survival in the first-line treatment of recurrent and/or metastatic SCCHN
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bonner JA et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567–578
Parkin DM et al. (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Argiris A et al. (2004) Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer 101: 2222–2229
Colevas AD (2006) Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 24: 2644–2652
Clavel M et al. (1994) Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol 5: 521–526
Forastiere AA et al. (1992) Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol 10: 1245–1251
Jacobs C et al. (1992) A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol 10: 257–263
Gibson MK et al. (2005) Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol 23: 3562–3567
León X et al. (2005) A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol 17: 418–424
Khuri FR et al. (2000) Treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck: current status and future directions. Semin Oncol 27: 25–33
Rubin Grandis J et al. (1997) Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene 15: 409–416
Shin DM et al. (1994) Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res 54: 3153–3159
Nozawa H et al. (2006) Small interfering RNA targeting epidermal growth factor receptor enhances chemosensitivity to cisplatin, 5-fluorouracil and docetaxel in head and neck squamous cell carcinoma. Cancer Sci 97: 1115–1124
Takes RP et al. (1998) Differences in expression of oncogenes and tumor suppressor genes in different sites of head and neck squamous cell. Anticancer Res 18: 4793–4800
Maurizi M et al. (1996) Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br J Cancer 74: 1253–1257
Rubin Grandis J et al. (1998) Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 90: 824–828
Lynch TJ et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139
Paez JG et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500
Lee JW et al. (2005) Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res 11: 2879–2882
Chung CH et al. (2006) Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol 24: 4170–4176
Temam S et al. (2007) Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol 25: 2164–2170
Mrhalova M et al. (2005) Epidermal growth factor receptor—its expression and copy numbers of EGFR gene in patients with head and neck squamous cell carcinomas. Neoplasma 52: 338–343
Sok JC et al. (2006) Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res 12: 5064–5073
Ke LD et al. (1998) Differential expression of epidermal growth factor receptor in human head and neck cancers. Head Neck 20: 320–327
Chen Z et al. (2000) Correlation of cisplatin sensitivity with differential alteration of EGFR expression in head and neck cancer cells. Anticancer Res 20: 899–902
Goldstein NI et al. (1995) Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1: 1311–1318
Baselga J (2001) The EGFR as a target for anticancer therapy—focus on cetuximab. Eur J Cancer 37 (Suppl 4): S16–S22
Ciardiello F and Tortora G (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 7: 2958–2970
Huang SM et al. (2002) Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther 1: 507–514
Kang X et al. (2007) High affinity Fc receptor binding and potent inhibition of antibody-dependent cellular cytotoxicity (ADCC) in vitro by anti-epidermal growth factor receptor antibody cetuximab [abstract #3041]. J Clin Oncol 25 (19S)
Kimura H et al. (2007) Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci 98: 1275–1280
Fan Z et al. (1993) Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res 53: 4637–4642
Brown D et al. (2000) Antiepidermal growth factor receptor antibodies augment cytotoxicity of chemotherapeutic agents on squamous cell carcinoma cell lines. Otolaryngol Head Neck Surg 122: 75–83
Baselga J et al. (2000) Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol 18: 904–914
Shin DM et al. (2001) Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res 7: 1204–1213
Baselga J et al. (2005) Phase ll multicenter study of the anti-epidermal growth factor receptor (EGFR) monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 23: 5568–5577
Herbst RS et al. (2005) Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol 23: 5578–5587
Vermorken JB et al. (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 25: 2171–2177
Burtness B et al. (2005) Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 23: 8646–8654
Bourhis J et al. (2006) Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 24: 2866–2872
Vermorken JB et al. (2007) Cetuximab extends survival of patients with recurrent or metastatic SCCHN when added to first line platinum based therapy—results of a randomized phase III (Extreme) study [abstract #6091]. J Clin Oncol 25 (16S)
Vermorken JB et al. (2008) Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer 112: 2710–2719
Hitt R et al. (2007) Phase II study of combination cetuximab and weekly paclitaxel in patients with metastatic/recurrent squamous cell carcinoma of head and neck (SCCHN): Spanish Head and Neck Cancer Group (TTCC) [abstract #6012]. J Clin Oncol 25 (18S)
Perez-Soler R (2003) Can rash associated with HER1/EGFR inhibition be used as a marker of treatment outcome. Oncology (Williston Park) 17: 23–28
ERBITUX (2008) Summary of Product Characteristics. [http://www.emea.europa.eu/humandocs/Humans/EPAR/erbitux/erbitux.htm] (Accessed September 2008)
Curran D et al. (2007) Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 25: 2191–2197
Cunningham D et al. (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351: 337–345
Xiong HQ et al. (2004) Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol 22: 2610–2616
Norum J (2006) Cetuximab in the treatment of metastatic colorectal cancer: a model-based cost-effectiveness analysis. J Chemother 18: 532–537
Starling N et al. (2007) Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 96: 206–212
Brown B et al. (2008) An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland and the United Kingdom. Value Health [10.1111/j.1524-4733.2007.00302.x]
Author information
Authors and Affiliations
Ethics declarations
Competing interests
J Bernier declared he is a consultant and is on the Advisory Board for Merck AG.
Rights and permissions
About this article
Cite this article
Bernier, J. Drug Insight: cetuximab in the treatment of recurrent and metastatic squamous cell carcinoma of the head and neck. Nat Rev Clin Oncol 5, 705–713 (2008). https://doi.org/10.1038/ncponc1228
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1228
This article is cited by
-
Single-center prospective study on the efficacy of nivolumab against platinum-sensitive recurrent or metastatic head and neck squamous cell carcinoma
Scientific Reports (2022)
-
Role of MTA1 in head and neck cancers
Cancer and Metastasis Reviews (2014)
-
Outcome of patients treated with palliative weekly Paclitaxel plus Cetuximab in recurrent head and neck cancer after failure of platinum-based therapy
European Archives of Oto-Rhino-Laryngology (2014)
-
Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma
Head & Neck Oncology (2009)
-
Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with 64Cu-DOTA-trastuzumab
European Journal of Nuclear Medicine and Molecular Imaging (2009)