Abstract
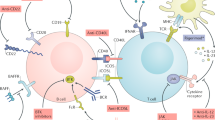
Inflammatory responses to cell-associated or tissue-associated immune complexes are key elements in the pathogenesis of several autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus and immune thrombocytopenic purpura. Effector cells, such as monocytes, macrophages and neutrophils, bind immune complexes in a process mediated by Fcγ receptors, and these cells then initiate inflammatory reactions that lead to tissue destruction. Rituximab is an anti-CD20 monoclonal antibody that suppresses inflammation effectively in autoimmune diseases. It was initially approved by the FDA for the treatment of B-cell lymphomas and later for rheumatoid arthritis refractory to anti-tumor necrosis factor therapies. Rituximab is hypothesized to suppress disease injury in autoimmune diseases by promoting rapid and long-term elimination of circulating and possibly lymphoid-tissue-associated B cells. We suggest, however, that a different mechanism may underlie much of the therapeutic action of rituximab in autoimmune diseases: binding of tens of thousands of rituximab−IgG molecules to B cells generates decoy sacrificial cellular immune complexes that efficiently attract and bind Fcγ receptor-expressing effector cells, which diminishes recruitment of these effector cells at sites of immune complex deposition and, therefore, reduces inflammation and tissue damage.
Key Points
-
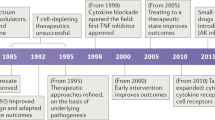
Rituximab has demonstrated therapeutic efficacy in patients with B-cell lymphomas or autoimmune diseases
-
The principal mechanism by which rituximab was postulated to decrease disease activity in autoimmune diseases was its ability to eliminate B cells and thus reduce autoantibody levels
-
Serological changes, however, have not accompanied positive clinical responses in patients treated with rituximab
-
In many autoimmune diseases, binding of tissue-associated immune complexes to monocytes or macrophages is an essential step in the initiation of inflammation and tissue injury
-
Blocking this immune-complex–effector-cell interaction can substantially reduce inflammation and disease pathology in animal models of autoimmune diseases
-
Rituximab-opsonized B cells may act as decoy immune complexes that effectively divert monocytes or macrophages from interactions with tissue-associated immune complexes, which provides an alternative mechanism to explain the therapeutic efficacy of rituximab in autoimmune disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grillo-Lopez A et al. (1999) Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol 26: 66–73
Edwards JCW et al. (2004) Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350: 2572–2581
Gottenberg JE et al. (2005) Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis 64: 913–920
Eisenberg R and Albert D (2005) B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol 2: 1–6
Cohen SB et al. (2006) Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 54: 2793–2806
Sfikakis PP et al. (2005) Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol 17: 550–557
Edwards JCW and Cambridge G (2006) B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol 6: 394–403
Looney RJ (2006) B cell-targeted therapy for rheumatoid arthritis. An update on the evidence. Drugs 66: 625–639
Reff ME et al. (1994) Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83: 435–445
Kennedy AD et al. (2004) Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol 172: 3280–3288
Cragg MS et al. (2005) The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun 8: 140–174
Lefebvre M-L et al. (2006) Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother 29: 388–397
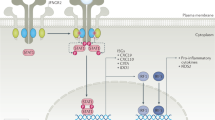
Takai T (2005) Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol 25: 1–18
Nimmerjahn F and Ravetch JV (2006) Fcγ receptors: old friends and new family members. Immunity 24: 19–28
Nimmerjahn F and Ravetch JV (2005) Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310: 1510–1512
Weng WK and Levy R (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 21: 3940–3947
Cartron G et al. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99: 754–758
Anolik JH et al. (2003) The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 48: 455–459
Edwards JCW and Cambridge G (1998) Rheumatoid arthritis: the predictable effect of small immune complexes in which antibody is also antigen. Br J Rheumatol 37: 126–130
Nimmerjahn F et al. (2005) FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23: 41–51
Shushakova N et al. (2002) C5a anaphylatoxin is a major regulator of activating versus inhibitory FcγRs in immune complex-induced lung disease. J Clin Invest 110: 1823–1830
Schmidt RE and Gessner JE (2005) Fc receptors and their interaction with complement in autoimmunity. Immunol Lett 100: 56–67
Kumar V et al. (2006) Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest 116: 512–520
Huber-Lang M et al. (2006) Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med 12: 682–687
Badger AM (2000) Antimacrophage therapies. In Rheumatoid arthritis: frontiers in pathogenesis and treatment, 539–549 (Eds Firestein GS et al.) New York: Oxford University Press
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423: 356–361
van Roon JAG et al. (2003) Selective elimination of synovial inflammatory macrophages in rheumatoid arthritis by an Fcγ receptor I-directed immunotoxin. Arthritis Rheum 48: 1229–1238
Wijngaarden S et al. (2004) A shift in the balance of inhibitory and activating Fcγ receptors on monocytes toward the inhibitory Fcγ receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum 50: 3878–3887
Samuelsson A et al. (2001) Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291: 484–486
Clynes R et al. (1998) Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279: 1052–1054
Marino M et al. (2000) Prevention of systemic lupus erythematosus in MRL/lpr mice by administration of an immunoglobulin-binding peptide. Nature Biotechnol 18: 735–739
Frank MM et al. (1979) Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med 300: 518–523
Salmon JE (2000) Abnormalities in immune complex clearance and Fcγ receptor function. In Dubois' Lupus Erythematosus, 219–242 (Eds Wallace DJ and Hahn BH) Baltimore: Williams and Wilkins
Manderson AP et al. (2004) The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22: 431–456
Zvaifler NJ (1973) The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol 16: 265–336
Ji H et al. (2002) Arthritis critically dependent on innate immune system players. Immunity 16: 157–168
Bruhns P et al. (2003) Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity 18: 573–581
Kaplan CD et al. (2005) Development of proteoglycan-induced arthritis is critically dependent on Fcγ receptor III expression. Arthritis Rheum 52: 1612–1619
O'Neill SK et al. (2005) Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol 174: 3781–3788
Anolik JH et al. (2004) Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 50: 3580–3590
Eisenberg R and Looney RJ (2005) The therapeutic potential of anti-CD20: what do B-cells do? Clin Immunol 117: 207–213
Sfikakis PP et al. (2005) Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand. Arthritis Rheum 52: 501–513
Leandro MJ et al. (2005) B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology (Oxford) 44: 1542–1545
Swaak AJG et al. (1986) Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis 45: 359–366
Looney RJ et al. (2004) B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50: 2580–2589
McLaughlin P (2001) Rituximab: perspective on single agent experience, and future directions in combination trials. Crit Rev Oncol Hematol 40: 3–16
Taylor RP et al. (1981) Studies of artificial cryoprecipitates containing anti-DNA antibody activity. Rheumatol Int 1: 1–6
Bussel JB et al. (1991) Intravenous anti-D treatment of immune thrombocytopenic purpura: analysis of efficacy, toxicity, and mechanism of effect. Blood 77: 1884–1893
Scaradavou A et al. (1997) Intravenous anti-D treatment of immune thrombocytopenic purpura: experience in 272 patients. Blood 89: 2689–2700
Stasi R et al. (2001) Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood 98: 952–957
Zaja F et al. (2003) The B-cell compartment as the selective target for the treatment of immune thrombocytopenias. Haematologica 88: 538–546
Song S et al. (2003) Monoclonal IgG can ameliorate immune thrombocytopenia in a murine model of ITP: an alternative to IVIG. Blood 101: 3708–3713
Siragam V et al. (2005) Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease? J Clin Invest 115: 155–160
Song S et al. (2005) Monoclonal antibodies that mimic the action of anti-D in the amelioration of murine ITP act by a mechanism distinct from that of IVIg. Blood 105: 1546–1548
Bowles JA and Weiner GJ (2005) CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Meth 304: 88–99
Schroder C et al. (2003) Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transplant Immunol 12: 19–28
Vugmeyster Y et al. (2003) Differential in vivo effects of rituximab on two B-cell subsets in cynomolgus monkeys. Int Immunopharmacol 3: 1477–1481
Vugmeyster Y et al. (2005) Depletion of B cells by a humanized anti-CD20 antibody PRO70769 in Macaca fascicularis. J Immunother 28: 212–219
Kennedy AD et al. (2003) An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood 101: 1071–1079
Uwatoko S et al. (1995) C1q-binding immunoglobulin G in MRL/I mice consists of immune complexes containing antibodies to DNA. Clin Immunol Immunopath 75: 140–146
Hamaguchi Y et al. (2005) The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol 174: 4389–4399
Gong Q et al. (2005) Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174: 817–826
Cardarelli PM et al. (2002) Binding to CD20 by anti-B1 antibody or F(ab')2 is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother 51: 15–24
Ravetch JV (2003) Fc receptors. In Fundamental Immunology, 685–700 (Ed Paul WE) Philadelphia: Lippincott Williams & Wilkins
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Taylor, R., Lindorfer, M. Drug Insight: the mechanism of action of rituximab in autoimmune disease—the immune complex decoy hypothesis. Nat Rev Rheumatol 3, 86–95 (2007). https://doi.org/10.1038/ncprheum0424
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0424
This article is cited by
-
Rituximab administration in pediatric patients with newly diagnosed acute lymphoblastic leukemia
Leukemia (2023)
-
Polymeric particle-based therapies for acute inflammatory diseases
Nature Reviews Materials (2022)
-
Genetics of ANCA-associated vasculitis: role in pathogenesis, classification and management
Nature Reviews Rheumatology (2022)
-
Low class–switched memory B cells predict the need for continued immunoglobulin replacement following B cell reconstitution after rituximab: a case series and review of the literature
Journal of Hematopathology (2020)
-
Rituximab to treat gemcitabine-induced hemolytic–uremic syndrome (HUS) in pancreatic adenocarcinoma: a case series and literature review
Cancer Chemotherapy and Pharmacology (2017)