Abstract
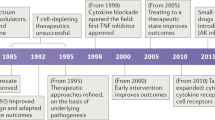
Advances in our understanding of the pathogenesis of rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosus have led to the emergence of immunoglobulin-based therapy as a major therapeutic force. Numerous monoclonal antibodies that target proinflammatory cytokines or their receptors (e.g. infliximab, adalimumab, tocilizumab, belimumab, HuMax-IL-15), and cell-surface or co-stimulatory molecules (e.g. rituximab) are either in clinical development or have been approved for clinical use. These antibodies are safe and effective in the long-term therapy of many rheumatic diseases. In addition, polyclonal immunoglobulins (intravenous immunoglobulin) obtained from pooled plasma from healthy blood donors are an effective therapeutic approach in certain rheumatic diseases. The mechanisms of action of monoclonal antibodies and intravenous immunoglobulin include cytolysis of target cells through complement or antibody-dependent cell-mediated cytotoxicity, induction of apoptosis of target cells, blockade of co-stimulatory molecules, and neutralization of pathogenic antibodies and soluble factors such as cytokines and their receptors, which ultimately lead to amelioration of the inflammatory process. The success of currently available therapeutic immunoglobulins has led to considerable interest in the identification of novel molecular therapeutic targets in rheumatic diseases.
Key Points
-
Immunoglobulin-based therapies, including therapeutic monoclonal antibodies and intravenous immunoglobulins (IVIg), have emerged as a major force in the immunotherapy of several rheumatic diseases
-
The first-generation therapeutic monoclonal antibodies against cell-surface molecules or soluble factors have proven effective in many rheumatic diseases
-
The mechanisms of action of therapeutic monoclonal antibodies include cytolysis of target cells, induction of apoptosis of target cells, blockade of co-stimulatory molecules, and/or neutralization of soluble factors and their receptors
-
Patients with rheumatic diseases could benefit from IVIg therapy since it behaves as a single drug with multiple targets
-
The mechanisms of action of IVIg are multiple and complex: some depend on the interaction between the Fc portion of infused IVIg and Fcγ receptors on target cells, others rely on the variable regions of IgG antibodies in the IVIg preparation
-
Our improved understanding of the pathogenesis of rheumatic diseases will lead to the discovery of new treatment strategies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Eisenberg R and Albert D (2006) B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol 2: 20–27
Edwards JC et al. (2004) Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350: 2572–2581
Leandro MJ et al. (2006) Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54: 613–620
Leandro MJ et al. (2002) An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 46: 2673–2677
Looney RJ et al. (2004) B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50: 2580–2589
Pijpe J et al. (2005) Rituximab treatment in patients with primary Sjögren's syndrome: an open-label phase II study. Arthritis Rheum 52: 2740–2750
Sfikakis PP et al. (2005) Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum 52: 501–513
Cambridge G et al. (2003) Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 48: 2146–2154
Cambridge G et al. (2006) B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum 54: 3612–3622
Popa C et al. (2007) Repeated B lymphocyte depletion with rituximab in rheumatoid arthritis over 7 yrs. Rheumatology (Oxford) 46: 626–630
Dorner T et al. (2006) Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther 8: R74
Reiff A (2005) A review of Campath in autoimmune disease: biologic therapy in the gray zone between immunosuppression and immunoablation. Hematology 10: 79–93
Isaacs JD et al. (1992) Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet 340: 748–752
Brett S et al. (1996) Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, Campath-1H. Immunology 88: 13–19
Grammer AC et al. (2003) Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154–CD40 interactions. J Clin Invest 112: 1506–1520
Huang W et al. (2002) The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum 46: 1554–1562
Boumpas DT et al. (2003) A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 48: 719–727
Kalunian KC et al. (2002) Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 46: 3251–3258
Weyand CM and Goronzy JJ (2006) T-cell-targeted therapies in rheumatoid arthritis. Nat Clin Pract Rheumatol 2: 201–210
Isaacs JD et al. (1996) A therapeutic human IgG4 monoclonal antibody that depletes target cells in humans. Clin Exp Immunol 106: 427–433
Mason U et al. (2002) CD4 coating, but not CD4 depletion, is a predictor of efficacy with primatized monoclonal anti-CD4 treatment of active rheumatoid arthritis. J Rheumatol 29: 220–229
Hepburn TW et al. (2003) Antibody-mediated stripping of CD4 from lymphocyte cell surface in patients with rheumatoid arthritis. Rheumatology (Oxford) 42: 54–61
Utset TO et al. (2002) Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol 29: 1907–1913
Feldmann M and Maini RN (2001) Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 19: 163–196
Lipsky PE et al. (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 343: 1594–1602
Quinn MA et al. (2005) Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 52: 27–35
Weinblatt ME et al. (2003) Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 48: 35–45
Antoni CE et al. (2005) Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 52: 1227–1236
Mease PJ et al. (2005) Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 52: 3279–3289
Schuerwegh AJ et al. (2003) Influence of therapy with chimeric monoclonal tumour necrosis factor-alpha antibodies on intracellular cytokine profiles of T lymphocytes and monocytes in rheumatoid arthritis patients. Rheumatology (Oxford) 42: 541–548
Mastroianni A et al. (2005) Cytokine profiles during infliximab monotherapy in psoriatic arthritis. Br J Dermatol 153: 531–536
Ehrenstein MR et al. (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 200: 277–285
Valencia X et al. (2006) TNF downmodulates the function of human CD4+ CD25hi T-regulatory cells. Blood 108: 253–261
Bartelds GM et al. (2006) High levels of human anti-human antibodies to adalimumab in a patient not responding to adalimumab treatment. Ann Rheum Dis 65: 1249–1250
Cheema GS et al. (2001) Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 44: 1313–1319
Seyler TM et al. (2005) BLyS and APRIL in rheumatoid arthritis. J Clin Invest 115: 3083–3092
Carter RH et al. (2005) Expression and occupancy of BAFF-R on B cells in systemic lupus erythematosus. Arthritis Rheum 52: 3943–3954
Furie R et al. (2003) Safety, pharmacokinetic and pharmacodynamic results of a phase 1 single and double-escalation study of Lymphostat-B (human monoclonal antibody to BLyS) in SLE patients [abstract #922]. Arthritis Rheum 48: S377
Stohl W et al. (2005) Belimumab (BmAb), a novel fully human monoclonal antibody to B-lymphocyte stimulator (BLyS), selectively modulates B-cell subpopulations and immunoglobulins in a heterogeneous rheumatoid arthritis subject population [abstract #1160]. Arthritis Rheum 52: S444
McInnes IB and Liew FY (2005) Cytokine networks—towards new therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol 1: 31–39
Nishimoto N et al. (2004) Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 50: 1761–1769
Yokota S et al. (2005) Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 52: 818–825
Baslund B et al. (2005) Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum 52: 2686–2692
Llorente L et al. (2000) Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum 43: 1790–1800
Kazatchkine MD and Kaveri SV (2001) Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345: 747–755
Dalakas MC (2004) Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 291: 2367–2375
Toubi E et al. (2005) High-dose intravenous immunoglobulins: an option in the treatment of systemic lupus erythematosus. Hum Immunol 66: 395–402
Braun-Moscovici Y and Furst DE (2003) Immunoglobulin for rheumatic diseases in the twenty-first century: take it or leave it? Curr Opin Rheumatol 15: 237–245
Dalakas MC et al. (1993) A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 329: 1993–2000
Raju R and Dalakas MC (2005) Gene expression profile in the muscles of patients with inflammatory myopathies: effect of therapy with IVIg and biological validation of clinically relevant genes. Brain 128: 1887–1896
Walter MC et al. (2000) High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J Neurol 247: 22–28
Sherer Y et al. (2000) Intravenous immunoglobulin therapy of antiphospholipid syndrome. Rheumatology (Oxford) 39: 421–426
Bucciarelli S et al. (2006) Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum 54: 2568–2576
Aries PM et al. (2005) Intravenous immunoglobulin therapy in vasculitis: speculation or evidence? Clin Rev Allergy Immunol 29: 237–245
Levy Y et al. (1999) A study of 20 SLE patients with intravenous immunoglobulin—clinical and serologic response. Lupus 8: 705–712
Boletis JN et al. (1999) Intravenous immunoglobulin compared with cyclophosphamide for proliferative lupus nephritis. Lancet 354: 569–570
Giannini EH et al. (1996) Intravenous immunoglobulin in the treatment of polyarticular juvenile rheumatoid arthritis: a phase I/II study. Pediatric Rheumatology Collaborative Study Group. J Rheumatol 23: 919–924
Takahashi Y et al. (2003) Benefit of IVIG for long-standing ataxic sensory neuronopathy with Sjögren's syndrome. IV immunoglobulin. Neurology 60: 503–505
Sherer Y and Shoenfeld Y (2006) Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol 2: 99–106
Pierangeli SS et al. (2001) Identification of an Fc gamma receptor-independent mechanism by which intravenous immunoglobulin ameliorates antiphospholipid antibody-induced thrombogenic phenotype. Arthritis Rheum 44: 876–883
Akilesh S et al. (2004) The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest 113: 1328–1333
Bruhns P et al. (2003) Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity 18: 573–581
Siragam V et al. (2006) Intravenous immunoglobulin ameliorates ITP via activating Fcgamma receptors on dendritic cells. Nat Med 12: 688–692
Lutz HU et al. (2004) Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood 103: 465–472
Bayry J et al. (2003) Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood 101: 758–765
Bayry J et al. (2003) Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-alpha present in serum from patients with systemic lupus erythematosus. Arthritis Rheum 48: 3497–3502
Brannagan TH et al. (1996) Complications of intravenous immune globulin treatment in neurologic disease. Neurology 47: 674–677
Jarius S et al. (2007) Intravenous immunoglobulins contain naturally occurring antibodies that mimic anti-neutrophil cytoplasmic antibodies and activate neutrophils in a TNF-alpha dependent and Fc-receptor independent way. Blood [doi:10.1182/blood-2005-12-019604]
Gregori L et al. (2004) Partitioning of TSE infectivity during ethanol fractionation of human plasma. Biologicals 32: 1–10
Ahmed AR et al. (2006) Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med 355: 1772–1779
Acknowledgements
Supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM) and Centre National de la Recherche Scientifique (CNRS), France; Laboratoire Français du Fractionnement et des Biotechnologies, Les Ulis, France; CSL Behring, Switzerland; Octapharma, Austria and Talecris, USA. We are grateful to Dr Peter J Spath for the conception of Figure 1; Professor Marinos C Dalakas for inspiring the conception of Figure 3; Professor F Tron, Dr HK Hariharan and Professor MW Weksler for critical review of the manuscript. Owing to space limitations, we could not cite all relevant published work; we do not mean to undermine the value of uncited studies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors received financial support from CSL Behring, Switzerland; Laboratoire Français du Fractionnement et des Biotechnologies, France; Switzerland; Octapharma, Austria and Talecris, USA for their research activities at INSERM Unité 681.
Rights and permissions
About this article
Cite this article
Bayry, J., Lacroix-Desmazes, S., Kazatchkine, M. et al. Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nat Rev Rheumatol 3, 262–272 (2007). https://doi.org/10.1038/ncprheum0481
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0481
This article is cited by
-
FCN1 (M-ficolin), which directly associates with immunoglobulin G1, is a molecular target of intravenous immunoglobulin therapy for Kawasaki disease
Scientific Reports (2017)
-
Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain–Barré syndrome patients
Immunologic Research (2014)
-
Treatment of Multifocal Motor Neuropathy
Current Treatment Options in Neurology (2014)
-
Multifocal Motor Neuropathy: Current Therapies and Novel Strategies
Drugs (2013)
-
Intravenous immunoglobulin therapy in rheumatic diseases
Nature Reviews Rheumatology (2011)