Abstract
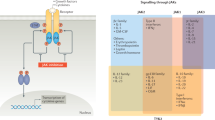
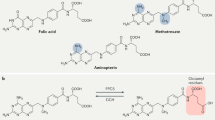
Angiogenesis inhibition, long studied in the treatment of malignancies, has begun to emerge as a potential therapeutic approach in managing inflammatory arthritis, particularly rheumatoid arthritis. The growth of new vessels is required for the development of the rheumatoid pannus, which then leads to extensive synovial inflammation and joint destruction. Vascular endothelial growth factor is the best studied mediator of angiogenesis, and several therapies have been developed that specifically target this molecule. Several other angiogenesis mediators, such as the angiopoietin−TIE system, hypoxia inducible factor and integrin αVβ3, as well as naturally occurring inhibitors of angiogenesis, are also being investigated as potential therapeutic targets. Additionally, there are a number of drugs, including paclitaxel, 2-methoxyestradiol and fumagillin analogs, that might have a role in inhibiting angiogenesis and, thus, in treating proliferative synovitis.
Key Points
-
Angiogenesis has a crucial role in the pathophysiology of inflammatory arthritis
-
In the synovium, angiogenesis is mediated by a variety of factors, including vascular endothelial growth factor, tumor necrosis factor, hypoxia, angiopoietin, angiostatin, endostatin and thrombospondin
-
Numerous drugs have been developed in the field of oncology that act to attenuate the effects of proangiogenic factors, thereby halting the progression of malignancy
-
These drugs, including vascular-endothelial-growth-factor inhibitors, microtubule disrupters and fumagillin analogs, are now being investigated as possible therapeutic options in proliferative synovitis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nagashima M et al. (2000) Imbalance in production between vascular endothelial growth factor and endostatin in patients with rheumatoid arthritis. J Rheumatol 27: 2339–2342
Fox RI and Kang H (1993) Arthritis and Allied Conditions vol. 1, 263–278 (Eds McCarty D and Koopman W) Philadelphia: Lea & Febiger
Feldmann et al. (1996) Rheumatoid arthritis. Cell 85: 307–310
Fearon U et al. (2003) Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol 30: 260–268
Peters CL et al. (2004) The transcription factors hypoxia-inducible factor 1alpha and Ets-1 colocalize in the hypoxic synovium of inflamed joints in adjuvant-induced arthritis. Arthritis Rheum 50: 291–296
Walsh DA (1999) Angiogenesis and arthritis. Rheumatology (Oxford) 38: 103–112
Middleton J et al. (2004) Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res Ther 6: 60–72
Clavel G et al. (2006) Relationship between angiogenesis and inflammation in experimental arthritis. Eur Cytokine Netw 17: 202–210
Dvorak HF et al. (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039
Veikkola T and Alitalo K (1999) VEGFs, receptors and angiogenesis. Semin Cancer Biol 9: 211–220
Bottomley MJ et al. (1999) Peripheral blood mononuclear cells from patients with rheumatoid arthritis spontaneously secrete vascular endothelial growth factor (VEGF): specific up-regulation by tumour necrosis factor-alpha (TNF-alpha) in synovial fluid. Clin Exp Immunol 117: 171–176
Ikeda M et al. (2000) Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol 191: 426–433
Scola MP et al. (2001) Expression of angiogenic factors in juvenile rheumatoid arthritis: correlation with revascularization of human synovium engrafted into SCID mice. Arthritis Rheum 44: 794–801
Berse B et al. (1999) Hypoxia augments cytokine (transforming growth factor-beta [TGF-beta] and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin Exp Immunol 115: 176–182
Cho ML et al. (2006) Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett 103: 159–166
Ryu S et al. (2006) IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clin Rheumatol 25: 16–20
Levy NS et al. (1998) Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 273: 6417–6423
Paleolog EM et al. (1998) Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum 41: 1258–1265
Strunk J et al. (2006) Anti-TNF-alpha antibody infliximab and glucocorticoids reduce serum vascular endothelial growth factor levels in patients with rheumatoid arthritis: a pilot study. Rheumatol Int 26: 252–256
Canete JD et al. (2004) Antiangiogenic effects of anti-tumor necrosis factor alpha therapy with infliximab in psoriatic arthritis. Arthritis Rheum 50: 1636–1641
Grosios K et al. (2004) Angiogenesis inhibition by the novel VEGF receptor tyrosine kinase inhibitor, PTK787/ZK222584, causes significant anti-arthritic effects in models of rheumatoid arthritis. Inflamm Res 53: 133–142
Holash J et al. (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99: 11393–11398
Hurwitz H et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342
Suri C et al. (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180
Davis S et al. (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87: 1161–1169
Maisonpierre PC et al. (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60
Debusk LM et al. (2003) Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum 48: 2461–2471
Gravallese EM et al. (2003) Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann Rheum Dis 62: 100–107
Scott BB et al. (2002) Constitutive expression of angiopoietin-1 and -2 and modulation of their expression by inflammatory cytokines in rheumatoid arthritis synovial fibroblasts. J Rheumatol 29: 230–239
Shahrara S et al. (2002) Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res 4: 201–208
Scott BB et al. (2005) TNF-alpha modulates angiopoietin-1 expression in rheumatoid synovial fibroblasts via the NF-kappa B signalling pathway. Biochem Biophys Res Commun 328: 409–414
Chen Y et al. (2005) Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum 52: 1585–1594
Jiang BH et al. (1996) Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271: 17771–17778
Liu LX et al. (2002) Stabilization of vascular endothelial growth factor mRNA by hypoxia-inducible factor 1. Biochem Biophys Res Commun 291: 908–914
Giatromanolaki A et al. (2003) Upregulated hypoxia inducible factor-1alpha and -2alpha pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 5: R193–R201
Hollander AP et al. (2001) Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum 44: 1540–1544
Jackson JR et al. (1997) Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin 1beta. J Rheumatol 24: 1253–1259
Yeo EJ et al. (2003) YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst 95: 516–525
Yeo EJ et al. (2004) New anticancer strategies targeting HIF-1. Biochem Pharmacol 68: 1061–1069
Mabjeesh NJ et al. (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3: 363–375
Escuin D et al. (2005) Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res 65: 9021–9028
Wilder RL (2002) Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann Rheum Dis 61 (Suppl 2): ii96–ii99
Johnson BA et al. (1993) Adhesion molecule expression in human synovial tissue. Arthritis Rheum 36: 137–146
Wu H et al. (1998) Stepwise in vitro affinity maturation of Vitaxin, an αv β3-specific humanized mAb. Proc Natl Acad Sci USA 95: 6037–6042
Badger AM et al. (2001) Disease-modifying activity of SB 273005, an orally active, nonpeptide alphavbeta3 (vitronectin receptor) antagonist, in rat adjuvant-induced arthritis. Arthritis Rheum 44: 128–137
Storgard CM et al. (1999) Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist. J Clin Invest 103: 47–54
Adams JD et al. (1993) Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr 15: 141–147
Brahn E et al. (1994) Regression of collagen-induced arthritis with taxol, a microtubule stabilizer. Arthritis Rheum 37: 839–845
Oliver SJ et al. (1994) Suppression of collagen-induced arthritis using an angiogenesis inhibitor, AGM-1470, and a microtubule stabilizer, taxol. Cell Immunol 157: 291–299
Fotsis T et al. (1994) The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 368: 237–239
Josefsson E and Tarkowski A (1997) Suppression of type II collagen-induced arthritis by the endogenous estrogen metabolite 2-methoxyestradiol. Arthritis Rheum 40: 154–163
Brahn E et al. (2006) Angiogenesis inhibition with 2-methoxyestradiol involutes collagen-induced arthritis and suppresses synovial VEGF and FGF-2 gene expression [abstract]. Ann Rheum Dis 65 (Suppl II): 80
Ingber D et al. (1990) Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 348: 555–557
de Bandt M et al. (2000) Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum 43: 2056–2063
Peacock DJ et al. (1992) Angiogenesis inhibition suppresses collagen arthritis. J Exp Med 175: 1135–1138
Peacock DJ et al. (1995) A novel angiogenesis inhibitor suppresses rat adjuvant arthritis. Cell Immunol 160: 178–184
Oliver SJ et al. (1995) Suppression of collagen-induced arthritis by an angiogenesis inhibitor, AGM-1470, in combination with cyclosporin: reduction of vascular endothelial growth factor (VEGF). Cell Immunol 166: 196–206
Nagashima M et al. (2002) Study of the mechanism involved in angiogenesis and synovial cell proliferation in human synovial tissues of patients with rheumatoid arthritis using SCID mice. Lab Invest 82: 981–988
Hannig G et al. (2007) Suppression of inflammation and structural damage in experimental arthritis through molecular targeted therapy with PPI-2458. Arthritis Rheum 56: 850–860
Bernier SG et al. (2004) A methionine aminopeptidase-2 inhibitor, PPI-2458, for the treatment of rheumatoid arthritis. Proc Natl Acad Sci USA 101: 10768–10773
O'Reilly MS et al. (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79: 315–328
Kim JM et al. (2002) Angiostatin gene transfer as an effective treatment strategy in murine collagen-induced arthritis. Arthritis Rheum 46: 793–801
Takahashi H et al. (2005) Adeno-associated virus vector-mediated anti-angiogenic gene therapy for collagen-induced arthritis in mice. Clin Exp Rheumatol 23: 455–461
Kato K et al. (2005) Human immunodeficiency virus vector-mediated intra-articular expression of angiostatin inhibits progression of collagen-induced arthritis in mice. Rheumatol Int 25: 522–529
O'Reilly MS et al. (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285
Kurosaka D et al. (2003) Inhibition of arthritis by systemic administration of endostatin in passive murine collagen induced arthritis. Ann Rheum Dis 62: 677–679
Matsuno H et al. (2002) Treatment with the angiogenesis inhibitor endostatin: a novel therapy in rheumatoid arthritis. J Rheumatol 29: 890–895
Yue L et al. (2004) Anti-adjuvant arthritis of recombinant human endostatin in rats via inhibition of angiogenesis and proinflammatory factors. Acta Pharmacol Sin 25: 1182–1185
Gotis-Graham I et al. (1997) Significant correlation between thrombospondin 1 and serine proteinase expression in rheumatoid synovium. Arthritis Rheum 40: 1780–1787
Koch AE et al. (1993) Localization of the angiogenesis inhibitor thrombospondin in human synovial tissues. Pathobiology 61: 1–6
Koch AE et al. (1998) Effects of thrombospondin-1 on disease course and angiogenesis in rat adjuvant-induced arthritis. Clin Immunol Immunopathol 86: 199–208
Jou IM et al. (2005) Thrombospondin 1 as an effective gene therapeutic strategy in collagen-induced arthritis. Arthritis Rheum 52: 339–344
D'Amato RJ et al. (1994) Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91: 4082–4085
Corral LG et al. (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163: 380–386
Komorowski J et al. (2006) Effect of thalidomide affecting VEGF secretion, cell migration, adhesion and capillary tube formation of human endothelial EA.hy 926 cells. Life Sci 78: 2558–2563
Oliver SJ et al. (1998) The effect of thalidomide and 2 analogs on collagen induced arthritis. J Rheumatol 25: 964–969
Hauschild A et al. (1997) Thalidomide therapy of established collagen-induced arthritis (CIA) not accompanied by an evident Th2 shift. Clin Exp Immunol 108: 428–431
Oliver SJ et al. (1999) Thalidomide analogue CC1069 inhibits development of rat adjuvant arthritis. Clin Exp Immunol 118: 315–321
Huizinga TW et al. (1996) An open study of pentoxyfylline and thalidomide as adjuvant therapy in the treatment of rheumatoid arthritis. Ann Rheum Dis 55: 833–836
Kroger H et al. (1996) Synergistic effects of thalidomide and poly (ADP-ribose) polymerase inhibition on type II collagen-induced arthritis in mice. Inflammation 20: 203–215
Gutierrez-Rodriguez O (1984) Thalidomide. A promising new treatment for rheumatoid arthritis. Arthritis Rheum 27: 1118–1121
Gutierrez-Rodriguez O et al. (1989) Treatment of refractory rheumatoid arthritis—the thalidomide experience. J Rheumatol 16: 158–163
Huang F et al. (2002) One-year open-label trial of thalidomide in ankylosing spondylitis. Arthritis Rheum 47: 249–254
Lehman TJ et al. (2004) Thalidomide for severe systemic onset juvenile rheumatoid arthritis: a multicenter study. J Pediatr 145: 856–857
Wei JC et al. (2003) Thalidomide for severe refractory ankylosing spondylitis: a 6-month open-label trial. J Rheumatol 30: 2627–2631
Tong Y et al. (2004) Anti-angiogenic effects of Shiraiachrome A, a compound isolated from a Chinese folk medicine used to treat rheumatoid arthritis. Eur J Pharmacol 494: 101–109
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E Brahn has declared that he has acted as a consultant and has received grants/research support from Entremed.
D Lainer-Carr declared no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Lainer-Carr, D., Brahn, E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Rev Rheumatol 3, 434–442 (2007). https://doi.org/10.1038/ncprheum0559
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0559
This article is cited by
-
Prodrug-based nanomedicines for rheumatoid arthritis
Discover Nano (2024)
-
Remodeling articular immune homeostasis with an efferocytosis-informed nanoimitator mitigates rheumatoid arthritis in mice
Nature Communications (2023)
-
Assessment of angiogenesis-related parameters in juvenile idiopathic arthritis-associated uveitis
Molecular Biology Reports (2022)
-
Vasoinhibin reduces joint inflammation, bone loss, and the angiogenesis and vasopermeability of the pannus in murine antigen-induced arthritis
Laboratory Investigation (2020)
-
Taxol alleviates collagen-induced arthritis in mice by inhibiting the formation of microvessels
Clinical Rheumatology (2019)