Abstract
Heterozygosity of the retinoblastoma gene Rb1 elicits tumorigenesis in susceptible tissues following spontaneous loss of the remaining functional allele. Inactivation of previously studied retinoblastoma protein (pRb) targets partially inhibited tumorigenesis in Rb1+/− mice1,2,3,4,5,6. Here we report that inactivation of pRb target Skp2 (refs. 7,8) completely prevents spontaneous tumorigenesis in Rb1+/− mice. Targeted Rb1 deletion in melanotrophs ablates the entire pituitary intermediate lobe when Skp2 is inactivated. Skp2 inactivation does not inhibit aberrant proliferation of Rb1-deleted melanotrophs but induces their apoptotic death. Eliminating p27 phosphorylation on T187 in p27T187A knock-in mice reproduces the effects of Skp2 knockout, identifying p27 ubiquitination by SCFSkp2 ubiquitin ligase as the underlying mechanism for Skp2's essential tumorigenic role in this setting. RB1-deficient human retinoblastoma cells also undergo apoptosis after Skp2 knockdown; and ectopic expression of p27, especially the p27T187A mutant, induces apoptosis. These results reveal that Skp2 becomes an essential survival gene when susceptible cells incur Rb1 deficiency.
Similar content being viewed by others
Main
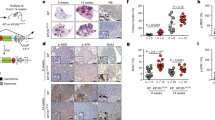
Skp2 binds T187-phosphorylated p27 for the SCFSkp2 ubiquitin ligase to ubiquitinate p27 (ref. 9). pRb binds Skp2 to interfere this binding and ubiquitination7. pRb-Skp2 binding also bridges Skp2 to the APC-Cdh1 ubiquitin ligase for Skp2 ubiquitination8. Because Skp2 is a target for the transcription factor E2F (refs. 10,11), pRb could repress Skp2 mRNA expression via E2F. Consistent with the above findings, Rb1+/− mice developed Rb1−/− pituitary tumors that had substantially increased amounts of Skp2 mRNA and protein along with decreased amounts of p27 protein (Fig. 1a,b).
(a) Expression of the indicated proteins in wild-type (WT) normal pituitary glands and pituitary tumors developed in Rb1+/−Skp2+/+ mice, determined by protein blot. (b) Amounts of Skp2 mRNA in pituitary glands and pituitary tumors (developed in Rb1+/− mice), determined by quantitative PCR (Q-PCR) normalized to the abundance of the enzyme GAPDH. (c) Incidence of pituitary and thyroid tumors at various stages in Rb1+/−Skp2+/+ and Rb1−/−Skp2−/− mice. P values are by Fisher's exact tests (various lesions were combined for analyses). (d) Kaplan-Meier survival analysis for the indicated mice. P value is by log rank test. One Rb1+/−Skp2−/− mouse died at 13 months, and one died at 16 months with macroscopically normal pituitary and thyroid glands. The causes of death were unclear, with a possible association with eye and skin lesions. (e) Kaplan-Meier survival analysis for the indicated mice treated with ENU.
To define the role of Skp2 in tumorigenesis in Rb1+/− mice, we generated cohorts of Rb1+/−Skp2+/+ and Rb1+/−Skp2−/− mice. Skp2 is not required for pituitary gland development (see Supplementary Fig. 1). Rb1+/− mice develop pituitary intermediate lobe melanotroph tumors with a well-defined course, from early atypical proliferates to foci, microscopic tumors and gross tumors (Supplementary Fig. 2a), resulting in death around 1 year of age12. At 6 months more than half of Rb1+/−Skp2+/+ mice had early atypical proliferates and foci (Fig. 1c). By 9 months one pituitary had a gross tumor, and most had foci and microscopic tumors. Later, all 27 Rb1+/−Skp2+/+ mice died between 10 and 15 months of age (Fig. 1d), all but one with gross pituitary tumors (Fig. 1c). In contrast, none of the Rb1+/−Skp2−/− mice had any sign of pituitary tumorigenesis at 6, 9 and 17 months, when healthy Rb1+/−Skp2−/− mice were killed.
Thyroid C-cell tumors develop with 50–70% penetrance in Rb1+/− mice. Among the same 27 Rb1+/−Skp2+/+ mice, 16 had gross thyroid tumors at death, and the dead mouse that lacked a pituitary tumor had an especially large thyroid tumor (Fig. 1c). About half of the remaining dead mice had microscopic thyroid tumors (Fig. 1c and Supplementary Fig. 2b). In contrast, all 29 Rb1+/−Skp2−/− mice had normal-appearing, tumor-free thyroid glands (Fig. 1c). Together with the lack of pituitary tumors, these results identify Skp2 as the first pRb target that is required for spontaneous tumorigenesis in Rb1+/− mice.
The above findings could reflect the fact that Skp2 plays a required role in the development of Rb1 mutant tumors or that Skp2 is generally required for tumorigenesis. To begin to investigate these possibilities, we treated Skp2+/+ and Skp2−/− mice with a tumorigenesis protocol using N-ethyl-N-nitrosurea (ENU) induction. This experiment demonstrated no difference in tumor development in the two genotypes, including survival (Fig. 1e) and tumor types and burdens (Supplementary Fig. 3a). Although Skp2 was frequently overexpressed in the tumors, its expression levels did not correlate with p27 protein levels (Supplementary Fig. 3b). Thus, Skp2 is not required for ENU-induced tumorigenesis.
Because spontaneous tumorigenesis in Rb1+/− mice requires the loss of the remaining Rb1 allele, it was possible that Skp2 inactivation prevented the second Rb1 mutation, rather than the growth of Rb1-deficient tumors. We next used a tissue-specific Rb1 deletion scheme involving a proopiomelanocortin promoter–Cre recombinase (POMC-Cre) fusion and loxP sites to artificially generate Rb1−/− pituitary intermediate lobe melanotrophs13. To determine whether Skp2 inactivation affects the efficiency of POMC-Cre–loxP-mediated recombination, we generated POMC-Cre;Rosa26R;Skp2+/+ and POMC-Cre;Rosa26R;Skp2−/− mice. We found that the POMC-Cre strain could induce Cre-loxP–mediated deletion in most of the intermediate lobe melanotrophs in both Skp2+/+ and Skp2−/− mice (Fig. 2a). Because the POMC promoter is also active in corticotrophs in the anterior lobe, scattered anterior lobe recombination events were detected in both strains of mice as well (Fig. 2a, v,vi).
(a) The POMC-Cre strain induced Cre-loxP–mediated excision in posterior and anterior lobes of Skp2+/+ and Skp2−/− mice. 'Rosa26R' indicates Rosa26-loxP-STOP-loxP-EYFP. Mice were examined at 4 weeks of age. EYFP expression was by fluorescence of frozen-sectioned samples. (b) Pituitary intermediate lobes of indicated mice at 7 weeks of age. Hematoxylin and eosin (H&E)-stained sections of various pituitaries are shown. Large inset in vii is enlarged view of areas marked by the small box. (c) Pituitary glands of the indicated mice at the indicated ages, examined as in a. Scale bar, 200 μm.
We then generated POMC-Cre;Rb1lox/loxSkp2+/+ and POMC-Cre;Rb1lox/loxSkp2−/− mice and examined their pituitary glands at 7 weeks of age. As expected13, POMC-Cre;Rb1lox/loxSkp2+/+ mice harbored dysplastic nodular lesions across the entire intermediate lobes (Fig. 2b, ii,vi). Unexpectedly, POMC-Cre;Rb1lox/loxSkp2−/− mice did not contain normal-appearing intermediate lobes as we predicted based on the lack of pituitary tumorigenesis in Rb1+/−Skp2−/− mice. Instead, the intermediate lobes of these mice were essentially absent, with only a single layer of lining cells separating the anterior and posterior lobes (Fig. 2b, iii,vii). The intermediate lobes of POMC-Cre;Rb1lox/loxSkp2+/− mice were also considerably thinner than normal (Fig. 2b, iv,viii). These results confirm that Skp2 inactivation blocks tumorigenesis and demonstrate that this effect was achieved not by reverting Rb1-deficient melanotrophs to normal cells but by eliminating them.
We traced the fate of Rb1 and Skp2 doubly deficient melanotrophs by generating POMC-Cre;Rosa26R;Rb1lox/loxSkp2+/+ and POMC-Cre;Rosa26R;Rb1lox/loxSkp2−/− mice and allowing them to age to 10–13 weeks. The intermediate lobes of POMC-Cre;Rosa26R;Rb1lox/loxSkp2+/+ mice, observed with hematoxylin stain and enhanced yellow fluorescent protein (EYFP) fluorescence (Fig. 2c, i,iii), were in more advanced stages of tumorigenesis than those at 7 weeks (compare with Fig. 2b, ii), whereas the intermediate lobes of POMC-Cre;Rosa26R;Rb1lox/loxSkp2−/− mice remained a single-cell layer (Fig. 2c, ii). Notably, the cells in this layer were EYFP positive (Fig. 2c, iv), suggesting that this single-cell layer environment could prevent death of Rb1 and Skp2 doubly deficient cells or that these cells escaped Rb1 deletion. We also found that Rb1 deletion in corticotrophs induced the presence of more corticotrophs in the anterior lobe, and combined deletion of Rb1 and Skp2 markedly reduced their numbers (Fig. 2a, v,vi, and c, iii,iv). This indicates that combined Rb1 and Skp2 deletion could eliminate corticotrophs as well as melanotrophs.
We next harvested the mice at earlier ages to investigate how the intermediate lobes were eliminated (Fig. 3). At postnatal day (P) 10 the intermediate lobes of both POMC-Cre;Rb1lox/loxSkp2+/+ and POMC-Cre;Rb1lox/loxSkp2−/− mice showed slightly higher cellularity than those of Rb1lox/lox mice (Fig. 3a, i,vi,xi, and data not shown). Expression of PCNA (encoded by an E2F target gene) and Ki67 (a proliferation marker) was readily observed in Rb1lox/lox melanotrophs, indicating the proliferative status of these cells at this age (Fig. 3a, ii,iii, and c). Deletion of Rb1 increased PCNA and Ki67 expression, consistent with deregulation of E2F and proliferation caused by pRb inactivation (Fig. 3a, vii,viii, and c). Skp2 inactivation reduced neither PCNA expression nor the aberrant proliferation of the Rb1-deficient cells (Fig. 3a, xii,xiii, and c), but it significantly increased TUNEL-positive intermediate lobe cells compared with Rb1lox/lox and POMC-Cre;Rb1lox/loxSkp2+/+ controls (Fig. 3a, iv,ix,xiv, and d).
(a,b) Various indicated mice at the ages of P10 (a) and 4 weeks (b) are presented. E2F deregulation is examined by PCNA expression, proliferation by Ki67 expression and apoptosis by TUNEL labeling. (c,d) Quantification of Ki67 (c) and TUNEL labeling (d) in intermediate lobes was performed with three pituitaries of each indicated genotype at the indicated ages. Rb1 genotypes indicate the outcome of Cre-loxP–mediated deletion in intermediate lobe. P values are by Student's t-test. Error bars, s.d. Scale bar, 200 μm.
At 4 weeks of age the cells in the intermediate lobes of POMC-Cre;Rb1lox/loxSkp2−/− mice maintained deregulated PCNA expression and proliferation and increased apoptosis (Fig. 3b–d). Whereas the aberrantly proliferating intermediate lobes of 4-week-old POMC-Cre;Rb1lox/loxSkp2+/+ mice had become more than twofold thicker than those of the Rb1lox/lox controls (Fig. 3b, i,vi), the proliferating yet apoptotic intermediate lobes of 4-week-old POMC-Cre;Rb1lox/loxSkp2−/− mice had become more than twofold thinner than normal (Fig. 3b, xi). Together, these findings indicate that Skp2 is required for the survival of aberrantly proliferating Rb1-deficient melanotrophs and that Rb1−/−Skp2−/− melanotroph apoptosis caused the elimination of the intermediate lobes in POMC-Cre;Rb1lox/loxSkp2−/− mice.
POMC-Cre;Rb1lox/lox mice allowed us to evaluate the effect of Skp2 on p27 expression during melanotroph tumorigenesis using immunohistochemical staining. Melanotrophs of Rb1lox/lox, POMC-Cre;Rb1lox/lox and POMC-Cre;Rb1lox/loxSkp2−/− mice at P10 had comparable nuclear p27 protein stains (Fig. 3a, v,x,xv). However, by 4 weeks p27 concentrations clearly decreased in melanotrophs of POMC-Cre;Rb1lox/lox mice (Fig. 3b, x) but were maintained in the melanotrophs of POMC-Cre;Rb1lox/loxSkp2−/− mice (Fig. 3b, xv), suggesting that Skp2 is required for the downregulation of p27 during melanotroph tumorigenesis following Rb1 deletion.
We next investigated how Skp2 inactivation led to the failure of p27 downregulation and whether this failure was responsible for the tumor-blocking effects of Skp2 inactivation. In vitro studies have established that Skp2 mediates p27 ubiquitination in the SCFSkp2 ubiquitin ligase after p27 is phosphorylated on T187. However, the in vivo role of this Skp2 function has remained unclear because of divergent findings from Skp2-null mice (in which all Skp2 functions are absent) and p27T187A knock-in mice (in which only Skp2's ability to mediate ubiquitination of T187-phosphorylated p27 is absent). Skp2-null mice showed p27 protein accumulation in certain tissues and smaller body sizes14, but p27T187A knock-in mice neither showed p27 protein accumulation nor phenocopied Skp2-null mice15. Thus, in vivo, Skp2's ability to mediate ubiquitination of T187-phosphorylated p27 does not figure importantly in its ability to regulate p27. Our previous finding that pRb inhibits Skp2-mediated p27 ubiquitination by interfering with Skp2 binding to T187-phosphorylated p27 (ref. 7) suggested that this Skp2 function may be deregulated and contribute to p27 protein reduction and tumorigenesis following Rb1 loss. To evaluate this prediction, we generated POMC-Cre,Rb1lox/loxp27T187A/T187A and the control Rb1lox/loxp27T187A/T187A mice and examined their pituitary intermediate lobes at 4, 7 and 11 weeks of age.
The intermediate lobes of Rb1lox/loxp27T187A/T187A mice appeared normal (Fig. 4a, i), consistent with the general lack of abnormality in p27T187A/T187A mice. Following POMC-Cre–mediated Rb1 deletion, intermediate lobes of POMC-Cre,Rb1lox/loxp27T187A/T187A mice at 4 weeks of age did not show the hyperplastic thickening observed in POMC-Cre,Rb1lox/lox mice (Fig. 3b, vi), but instead had regional thinning (Fig. 4a, ii). The thinning of the intermediate lobe became more widespread by 7 weeks of age (Fig. 4a, iii), and by the age of 11 weeks the entire intermediate lobes were only two to three cell layers thick (Fig. 4a, iv). The nature of the T187A knock-in mutation (blocking T187 phosphorylation–dependent ubiquitination of p27 by SCFSkp2) predicted that the tumor-blocking effects observed in p27T187A/T187A homozygous mice should also occur in p27T187A/+ heterozygous mice, though potentially to a smaller extent. Results shown in Figure 4b confirm this prediction.
(a) Intermediate lobe morphology, PCNA expression, Ki67 and TUNEL labeling, and p27 expression were examined at the indicated ages. (b) Intermediate lobe morphology and PCNA expression after Rb1 deletion in p27T187A/+ mice at 7 and 11 weeks of age. (c,d) Quantification of Ki67 (c) and TUNEL labeling (d) in a. P values are by Student's t-test. Error bars, s.d. Scale bar, 200 μm.
Similar to the effects of Skp2 knockout in Rb1-deficient melanotrophs, p27T187A knock-in did not reduce the deregulated expression of PCNA and proliferation (Fig. 4a, v–vii,ix–xi, and c), but it increased apoptosis (Fig. 4a, xii–xiv, and b). These effects were also observed in the presence of one allele of p27T187A (Fig. 4b–d and data not shown). Finally, the reduced p27 expression in melanotrophs in 4-week-old POMC-Cre,Rb1lox/lox mice (Fig. 3b, x) occurred neither in melanotrophs in either 4- or 7-week-old POMC-Cre,Rb1lox/loxp27T187A/T187A mice (Fig. 4a, xv–xvii) nor in 7-week-old POMC-Cre,Rb1lox/loxp27T187A/+ mice (data not shown). Together these results suggest that the T187 phosphorylation–dependent ubiquitination of p27 by the SCFSkp2 ubiquitin ligase underlies Skp2's essential role in pituitary tumorigenesis following Rb1 loss, and that the apoptotic ablation of melanotrophs in POMC-Cre;Rb1lox/loxSkp2−/− mice could be explained by a proapoptotic effect of p27 in these cells16.
Notably, p27T187A knock-in is not equivalent to Skp2 knockout, because the intermediate lobes of POMC-Cre,Rb1lox/loxSkp2−/− mice thinned to a greater degree and with faster kinetics than those in POMC-Cre,Rb1lox/loxp27T187A/T187A mice (for example, compare Fig. 2b, iii,vii with Fig. 4a, iii,iv). Skp2 has a growing list of potential substrates in addition to p27 and can support cancer cell survival by protecting cyclin A from inhibition by p27 and p21 (ref. 17) and by blocking p53 activation by p300 (ref. 18). Further studies will be required to determine the roles of these additional mechanisms.
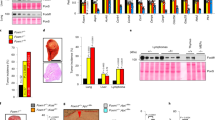
We next investigated whether the survival function of Skp2 revealed with mouse models was applicable to human tumors that develop as a result of Rb1 mutations. Because retinoblastoma is the main tumor that is associated with Rb1 deficiency in humans, we examined the effect of Skp2 knockdown in retinoblastoma cells. We found that knockdown of Skp2 (Fig. 5a) significantly inhibited retinoblastoma cell proliferation (Fig. 5b). Skp2 knockdown induced apoptosis, as measured by sub-G1 DNA content and TUNEL staining, but did not diminish S-phase population, as measured by FACS (Fig. 5c,d). The apoptotic effects of Skp2 knockdown were evident both in the established Y79 cell line and in early-passage RB177 cells.
(a–e) Y79 and RB177 cells infected with lentiviruses expressing short hairpin RNAs (shRNAs) targeting Skp2. Two independent Skp2 shRNAs and a scrambled shRNA control (Scrm) were used as indicated. After drug selection, infected cells were evaluated for Skp2 mRNA by quantitative reverse transcriptase PCR (a), cell proliferation by counting live cells (b), cell cycle profile by FACS (c), apoptosis by TUNEL staining (d) and p27 expression by protein immunoblotting, with Cdk2 as a loading control (e). (f–i) Y79 and RB177 cells infected with BE-GFP lentiviral vector encoding p27 or p27T187A. Infected cells were evaluated for p27 expression (f), cell proliferation (g), cell cycle profile (h) and TUNEL staining (i). (j,k) Y79 cells transduced with BE-GFP vector or BE-GFP-RB, followed 2 d later by transduction with Skp2 shRNA or scrambled shRNA control (j) or with BE-GFP or BE-GFP-p27T187A (k), and evaluated cells with sub-G1 DNA content. Averages with s.d. are shown. Asterisks indicate P < 0.05 relative to applicable controls. (l) A new model of tumorigenesis after Rb1 loss. Two consecutive arrows suggest the presence of multiple steps between them.
As expected, Skp2 knockdown induced accumulation of p27 in these human retinoblastoma cells (Fig. 5e). Moreover, ectopic expression of p27 was able to inhibit proliferation and induce apoptosis (Fig. 5f–i) similar to the effects of Skp2 knockdown. Notably, the mutant p27T187A was considerably more potent in inhibiting proliferation and inducing apoptosis, consistent with our findings from p27T187A knock-in mice. Restoration of pRb function largely prevented apoptosis induced either by Skp2 knockdown or by ectopic p27 expression (Fig. 5j,k), even though the modest pRb concentrations slowed but did not entirely block cell proliferation (data not shown), suggesting that lack of pRb rendered the retinoblastoma cells dependent on Skp2 and sensitive to aberrantly expressed p27.
We recently showed that MDM2 has essential roles in proliferation and survival of retinoblastoma cells and that knockdown of p14Arf diminished the requirement for MDM2 (ref. 19). In similar experiments we found that knockdown of p14Arf did not mitigate the effects of Skp2 knockdown, suggesting that p14Arf is not a critical target of Skp2 in these cells (Supplementary Fig. 4).
Before the current study, inactivation of previously studied pRb targets delayed tumorigenesis in Rb1+/− mice accompanied by reduced tumor cell proliferation1,2,3,4,5,6. In contrast, our study reveals that inactivation of Skp2 did not reduce deregulated proliferation of Rb1−/− cells but induced apoptosis, which completely prevented tumorigenesis. Our findings add a survival arm to the pRb-E2F model of pRb function, in which pRb loss not only deregulates E2F to result in aberrant proliferation and apoptosis through various E2F target genes but also deregulates the SCFSkp2-p27T187p p27 ubiquitination mechanism to downregulate p27 to provide survival support for the aberrantly proliferating pRb-deficient cells (Fig. 5l). When this mechanism is disrupted, either by inactivation of Skp2 or by blocking of p27 T187 phosphorylation, the outcome of pRb loss becomes cell death, revealing that Rb1 and Skp2 mutations are synthetically lethal to susceptible cells. The above model predicts that Skp2 is a potentially effective drug target to prevent and treat pRb-deficient tumors. Because our data imply that the p27T187 phosphorylation–dependent function of Skp2 is required for tumorigenesis following pRb loss, yet is not needed for normal development15, therapeutic targeting of Skp2 can focus on the p27T187-dependent function of Skp2 or p27T187 phosphorylation.
Methods
Mice.
Rb1+/− mice and Skp2+/− mice have been described elsewhere12,14. Mouse strain background is as follows. Skp2+/− mice on mixed C57BL/6J×129Sv strain background were backcrossed to C57BL/6J strain mice four times, and Rb1+/− mice on mixed C57BL/6J×129Sv strain background were backcrossed to C57BL/6J mice once. Rb1+/−Skp2+/− mice were then generated from these mice and were used to generate littermate Rb1+/−Skp2+/+ and Rb1+/−Skp2−/− mice. Our Rb1+/− mice may therefore show a slower tumor development kinetics than Rb1+/− mice with equal contributions from C57BL/6J and 129Sv strain background20. Rb1-heterozygous mice were genotyped according to a published protocol12. POMC-Cre transgenic mice were genotyped as described21. Primers for genotyping Skp2+/− mice, Rb1lox/lox mice22, Rosa26R(YFP) mice23 and p27T187A knock-in mice15 are listed in Supplementary Table 1.
The animals studied for ENU mutagenesis were C57BL/6J×129Sv hybrid strain littermate mice from Skp2 heterozygous crosses. Skp2+/+ and Skp2−/− mice were injected intraperitoneallywith ENU (0.5 mmol per g body weight) at P15 ± 2 d as described24. Mice were killed at the first sign of morbidity, which included abdominal swelling, hunched posture and rapid breathing. Complete necropsies of all internal organs were performed including size measurement of tumors.
All mouse study protocols were approved by the Albert Einstein College of Medicine Animal Institute.
Protein blot and reverse transcriptase–PCR analyses.
Normal pituitaries, fractions of gross pituitary tumors and fractions of ENU-induced tumors were snap-frozen in ethanol–dry ice and stored in −80 °C. For protein blot, frozen tissues were homogenized with Dounce glass homogenizer in tissue lysis buffer (50 mM HEPES, pH 7.2, 150 mM NaCl, 1 mM EDTA, 0.1% Tween-20, 1 mM dithiothreitol and standard protease inhibitors). Tissue debris was removed by centrifugation for 10 min at 14,000 r.p.m. in an Eppendorf Centrifuge 5415C (F-45-18-11 rotor) at 4 °C. Protein concentrations of the extracts were determined by Bio-Rad protein assay kit, and equal amounts of protein samples were loaded on 10% SDS gels and blotted onto polyvinylidene fluoride membrane. Antibodies to Skp2 (H435), p27 (C-19), cyclin A (C-19), cyclin E (M-20) and Cdk2 (C-19) were from Santa Cruz Biotechnology.
For quantitative PCR (Q-PCR), tissue RNA was extracted by Trizol reagent (Invitrogen). Total RNA was treated with RQ1 DNase (Promega) at 37 °C for 30 min, and RQ1 was denatured at 65 °C for 20 min. T7 oligonucleotides and SuperScript II (Invitrogen) were used for the synthesis of the first-strand cDNA at 42 °C for 60 min. The PCR primers for mSkp2 and mGAPDH are listed in Supplementary Table 1. SYBR Green PCR Master Mix (4309155; ABI) and the standard program of ABI Prism 7000 were used for Q-PCR amplification.
Immunohistochemistry staining and frozen sectioning for fluorescence detection.
Paraffin sections were stained with Histomouse-plus kit (Zymed) with antibodies to PCNA (PC10) and p27 (C-19) from Santa Cruz Biotechnology, and to BrdU (Ab-2) from Calbiochem, and Ki67 as primary antibody (1 μg/ml). TUNEL staining was performed with the reagents and instructions of Apoptosis Detection Kit (S7101) from Chemicon.
Pituitaries were fixed in 4% paraformaldehyde, 10% glucose in PBS for 30 min and embedded in Tissue Freezing Medium (H-TFM; Triangle Biomedical Sciences) on dry ice for frozen sectioning. After fluorescence photography, slides were counterstained by hematoxylin.
Lentivirus infection and analysis of human retinoblastoma cells.
Y79 cells were purchased from the American Type Culture Collection, and RB177 cells were derived from a human retinoblastoma and passaged for approximately 2 months, with no evidence of a crisis phase, before the knockdown analyses19. Skp2 shRNAs were delivered by pLKO constructs TRCN0000007530 and TRCN0000007534 (Open Biosystems) and were compared to a pLKO encoding a nonsilencing control shRNA (Addgene). RB177 cells with constitutive CDKN2AARF-null and pLKO-transduced controls were as described19. pRb, p27 and p27T187A were delivered using the bidirectional BE-GFP vector25. BE-GFP-p27+3′ and BE-GFP-p27T187A+3′ were produced by inserting a XmaI-XbaI fragment of pCS+p27 and pCS+p27(T187A)26 extending from the p27 coding region to the 3′ UTR between the corresponding XmaI site and a vector XbaI site of BE-GFP-p27 (ref. 25). BE-GFP-Rb was as described25. Cells were cultured, infected and analyzed as described19.
Statistical analysis.
In the survival analysis, difference in Kaplan-Meier survival curves was analyzed by log-rank test (JMP Software). Differences in gross tumor incidence and incidence of microscopic lesions in macroscopically normal pituitary and thyroid glands were analyzed by Fisher's exact test (MedCalc Software). Differences in TUNEL-labeling indices between Rb1lox/lox;POMC-Cre;Skp2+/+ and Rb1lox/lox;POMC-Cre;Skp2−/− intermediate lobes and between Rb1lox/lox;POMC-Cre;p27+/+ and Rb1lox/lox;POMC-Cre;p27T187A/T187A intermediate lobes were analyzed by Student's t-test (MedCalc Software).
References
Yamasaki, L. et al. Loss of E2F–1 reduces tumorigenesis and extends the lifespan of Rb1+/− mice. Nat. Genet. 18, 360–364 (1998).
Ziebold, U., Lee, E.Y., Bronson, R.T. & Lees, J.A. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol. Cell. Biol. 23, 6542–6552 (2003).
Lee, E.Y. et al. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell 2, 463–472 (2002).
Lasorella, A., Rothschild, G., Yokota, Y., Russell, R.G. & Iavarone, A. Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol. Cell. Biol. 25, 3563–3574 (2005).
Takahashi, C. et al. Nras loss induces metastatic conversion of Rb1-deficient neuroendocrine thyroid tumor. Nat. Genet. 38, 118–123 (2006).
Takahashi, C., Contreras, B., Bronson, R.T., Loda, M. & Ewen, M.E. Genetic interaction between Rb and K-ras in the control of differentiation and tumor suppression. Mol. Cell. Biol. 24, 10406–10415 (2004).
Ji, P. et al. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell 16, 47–58 (2004).
Binne, U.K. et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat. Cell Biol. 9, 225–232 (2007).
Frescas, D. & Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat. Rev. Cancer 8, 438–449 (2008).
Zhang, L. & Wang, C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene 25, 2615–2627 (2005).
Yung, Y., Walker, J.L., Roberts, J.M. & Assoian, R.K.A. Skp2 autoinduction loop and restriction point control. J. Cell Biol. 178, 741–747 (2007).
Jacks, T. et al. Effects of an Rb mutation in the mouse. Nature 359, 295–300 (1992).
Vooijs, M., van der Valk, M., te Riele, H. & Berns, A. Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene 17, 1–12 (1998).
Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000).
Malek, N.P. et al. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413, 323–327 (2001).
Carneiro, C. et al. p27 deficiency desensitizes Rb−/− cells to signals that trigger apoptosis during pituitary tumor development. Oncogene 22, 361–369 (2003).
Ji, P., Sun, D., Wang, H., Bauzon, F. & Zhu, L. Disrupting Skp2-cyclin A interaction with a blocking peptide induces selective cancer cell killing. Mol. Cancer Ther. 6, 684–691 (2007).
Kitagawa, M., Lee, S.H. & McCormick, F. Skp2 suppresses p53-dependent apoptosis by inhibiting p300. Mol. Cell 29, 217–231 (2008).
Xu, X.L. et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell 137, 1018–1031 (2009).
Leung, S.W. et al. A dynamic switch in Rb+/− mediated neuroendocrine tumorigenesis. Oncogene 23, 3296–3307 (2004).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–891 (2004).
Sage, J., Miller, A.L., Perez-Mancera, P.A., Wysocki, J.M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Timmerbeul, I. et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc. Natl. Acad. Sci. USA 103, 14009–14014 (2006).
Cobrinik, D., Francis, R.O., Abramson, D.H. & Lee, T.C. Rb induces a proliferative arrest and curtails Brn-2 expression in retinoblastoma cells. Mol. Cancer 5, 72 (2006).
Sheaff, R.J., Groudine, M., Gordon, M., Roberts, J.M. & Clurman, B.E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11, 1464–1478 (1997).
Acknowledgements
We thank J. Cui for expert technical assistance, R. Mahmood for help with tissue preparation and histological analysis, D. Abramson and S. Jhanwar for support of retinoblastoma cell analysis, A. Koff for comments on the manuscript and A. Burns and W. Zhang for encouragement. We are grateful to T. Jacks for providing Rb1+/− mice (from L. Yamasaki and A. Iavarone) and Rb1lox/lox mice, B. Lowell and S. Chua for POMC-Cre mice, J. Roberts for p27T187A knock-in mice and F. Costantini for Rosa26YFP mice (from J. Pollard). This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and National Cancer Institute of the US National Institutes of Health to L.Z. Albert Einstein Comprehensive Cancer Research Center and Liver Research Center provided core facility support. F.B. was supported by the Training Program in Cellular and Molecular Biology and Genetics (T32 GM007491) at the Albert Einstein College of Medicine. L.Z. is a recipient of the Irma T. Hirschl Career Scientist Award.
Author information
Authors and Affiliations
Contributions
H.W., P.J., F.B., D.S. and L.Z. designed and performed experiments with mice mutant for Rb1, Skp2, p27 or targeted deletion of Rb1. J.L. and R.S.S. performed pathology studies. D.C. and X.X. designed and performed analyses of retinoblastoma cells; H.W. performed protein blot experiments. K.N. and K.I.N. provided Skp2+/− mice. H.W., J.L., D.C. and L.Z. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 885 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Bauzon, F., Ji, P. et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat Genet 42, 83–88 (2010). https://doi.org/10.1038/ng.498
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.498
This article is cited by
-
Inhibitors Targeting the F-BOX Proteins
Cell Biochemistry and Biophysics (2023)
-
Akt: a key transducer in cancer
Journal of Biomedical Science (2022)
-
Targeting the untargetable: RB1-deficient tumours are vulnerable to Skp2 ubiquitin ligase inhibition
British Journal of Cancer (2022)
-
Functional genomics identifies new synergistic therapies for retinoblastoma
Oncogene (2020)
-
Modeling germline mutations in pineoblastoma uncovers lysosome disruption-based therapy
Nature Communications (2020)