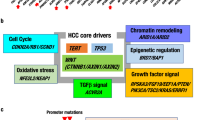
Abstract
Hepatocarcinogenesis is a slow process during which genomic changes progressively alter the hepatocellular phenotype to produce cellular intermediates that evolve into hepatocellular carcinoma. During the long preneoplastic stage, in which the liver is often the site of chronic hepatitis, cirrhosis, or both, hepatocyte cycling is accelerated by upregulation of mitogenic pathways, in part through epigenetic mechanisms. This leads to the production of monoclonal populations of aberrant and dysplastic hepatocytes that have telomere erosion and telomerase re-expression, sometimes microsatellite instability, and occasionally structural aberrations in genes and chromosomes. Development of dysplastic hepatocytes in foci and nodules and emergence of hepatocellular carcinoma are associated with the accumulation of irreversible structural alterations in genes and chromosomes, but the genomic basis of the malignant phenotype is heterogeneous. The malignant hepatocyte phenotype may be produced by the disruption of a number of genes that function in different regulatory pathways, producing several molecular variants of hepatocellular carcinoma. New strategies should enable these variants to be characterized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ferlay, J., Bray, F., Pisani, P. & Parkin, D.M.J. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0., IARC CancerBase No. 5 (Lyon, IARC Press, 2001).
Parkin, D.M., Bray, F.I. & Devesa, S.S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 37, S4–S66 (2001).
Grisham, J.W. Molecular genetic alterations in primary hepatocellular neoplasms: hepatocellular adenoma, hepatocellular carcinoma, and hepatoblastoma. In The Molecular Basis of Human Cancer (eds Coleman, W.B. & Tsongalis, G.J.) 269–346 (Humana Press, Totowa, New Jersey, 2001).
Bosch, F.X., Ribes, J. & Borràs, J. Epidemiology of primary liver cancer. Semin. Liver Dis. 19, 271–285 (1999).
Buendia, M.A. Genetics of hepatocellular carcinoma. Semin. Cancer Biol. 10, 185–200 (2000).
Bréchot, C. Molecular mechanisms of hepatitis B and C related to liver carcinogenesis. Hepatogastroenterology 45 (Suppl 3), 1189–1196 (1998).
Diao, J., Garces, R. & Richardson, C.D. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis (Survey). Cytokine Growth Factor Rev. 12, 189–205 (2001).
Smela, M.E., Currier, S.S., Bailey, E.A. & Essigmann, J.M. The chemistry and biology of aflatoxin B1: from mutational spectrometry to carcinogenesis. Carcinogenesis 22, 535–545 (2001).
Tong, M.J., El-Farra, N.S., Reikes, A.R. & Co, R.L. Clinical outcomes after transfusion-associated hepatitis C. New Engl. J. Med. 332, 1463–1466 (1995).
Takano, S., Yokosuka, O., Imazeki, F., Tagawa, M. & Omata, M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology 21, 650–655 (1995).
Chu, C.M. Natural history of chronic hepatitis B infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J. Gastroenterol. Hepatol. 15 (Suppl) E25–E30 (2000).
Oka, H. et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology 12, 680–687 (1990).
Ikeda, K. et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 18, 47–53 (1993).
Kato, Y. et al. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Analysis of infectious hepatitis viruses. Cancer 74, 2234–2238 (1994).
del Olmo, J.A. et al. Incidence and risk factors for hepatocellular carcinoma in 967 patients with cirrhosis. J. Cancer Res. Clin. Oncol. 124, 560–564 (1998).
Chiaramonte, M. et al. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer 85, 2132–2137 (1999).
Takayama, T. et al. Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet 336, 1150–1153 (1990).
Seki S. et al. Outcomes of dysplastic nodules in human cirrhotic liver: a clinicopathological study. Clin. Cancer Res. 6, 3469–3473 (2000).
Kawakita, N. et al. Analysis of proliferating hepatocytes using a monoclonal antibody against proliferating cell nuclear antigen/cyclin in embedded tissues from various liver diseases fixed in formaldehyde. Am. J. Pathol. 140, 513–520 (1992).
Schwienbacher, C. et al. Gain of imprinting at chromosome 11p15: a pathogenetic mechanism identified in human hepatocarcinoma. Proc. Natl Acad. Sci. USA 97, 5445–5449 (2000).
Soini, Y., Virkajärvi, N., Lehto, V.P. & Pääkkö, P. Hepatocellular carcinoma with a high proliferative index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br. J. Cancer 73, 1025–1030 (1996).
Theise, N.D. et al. Low proliferative activity in macroregenerative nodules: evidence for an alternate hypothesis concerning human hepatocarcinogenesis. Liver 16, 134–139 (1996).
Paradis, V., Laurendeau. I., Vidaud, M. & Bedossa, P. Clonal analysis of macronodules in cirrhosis. Hepatology 28, 953–958 (1998).
Ochai, T., Urata, Y., Yamano, T., Yamagishi, H. & Ashihara, T. Clonal expansion in evolution of chronic hepatitis to hepatocellular carcinoma as seen at an X-chromosome locus. Hepatology 31, 615–621 (2000).
Paradis, V. et al. Clonal analysis of micronodules in virus C-induced liver cirrhosis using laser capture microdissection (LCM) and HUMARA assay. Lab. Invest. 80, 1553–1559 (2000).
Okuda, T. et al. Clonal analysis of hepatocellular carcinoma and dysplastic nodule by methylation pattern of X-chromosome-linked human androgen receptor gene. Cancer Lett. 164, 91–96 (2001).
Kitamoto, M. & Ide, T. Telomerase activity in precancerous hepatic nodules. Cancer 85, 245–248 (1999).
Takaishi, H. et al. Precancerous hepatic nodules had significant levels of telomerase activity determined by sensitive quantitation using hybridization protection assay. Cancer 88, 312–317 (2000).
Toshikuni, N. et al. Expression of telomerase-associated protein 1 and telomerase reverse transcriptase in hepatocellular carcinoma. Br. J. Cancer 82, 833–837 (2000).
Liu, Y.W., Chang, K.J. & Liu, Y.C. DNA hypomethylation of proliferating cell nuclear antigen gene in human hepatocellular carcinoma is not due to cell proliferation. Cancer Lett. 70, 189–196 (1993).
Shen, L. et al. Correlation between DNA methylation and pathological changes in human hepatocellular carcinoma. Hepatogastroenterology 45, 1753–1759 (1998).
Kanai, Y. et al. DNA hypermethylation at the D17S5 locus and reduced HIC-1 mRNA expression are associated with hepatocarcinogenesis. Hepatology 29, 703–709 (1999).
Nagai, H. et al. A novel sperm-specific hypomethylation sequence is a demethylation hotspot in human hepatocellular carcinoma. Gene 237, 15–20 (1999).
Kanai, Y., Ushijima, S., Tsuda, H., Sakamoto, M. & Hirohashi, S. Aberrant DNA methylation precedes loss of heterozygosity on chromosome 16 in chronic hepatitis and liver cirrhosis. Cancer Lett. 148, 73–80 (2000).
JinBaek, M. et al. p16 is a major inactivation target in hepatocellular carcinoma. Cancer 89, 60–68 (2000).
Kondo, Y. et al. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis—a comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology 32, 970–979 (2000).
Takai, D., Yagi, Y., Habib, N., Sugimura, T. & Ushijima, T. Hypomethylation of LINE1 retrotransposon in human hepatocellular carcinomas, but not in surrounding liver cirrhosis. Jpn. J. Clin. Oncol. 30, 306–309 (2000).
Tchou, J.C. et al. GSTP1 CpG island DNA hypermethylation in hepatocellular carcinoma. Int. J. Oncol. 16, 663–676 (2000).
Kaneto, H. et al. Detection of hypermethylation of the p16INK4A gene promoter in chronic hepatitis and cirrhosis associated with hepatitis B or C virus. Gut 48, 372–377 (2001).
Lin, C.H. et al. Genome-wide hypomethylation in hepatocellular carcinogenesis. Cancer Res. 61, 4238–4243 (2001).
Saito, Y. et al. Expression of mRNA for DNA methyltransferases and methyl-CpG-binding proteins and DNA methylation status on CpG islands and pericentromeric satellite regions during human hepatocarcinogenesis. Hepatology 33, 561–568 (2001).
Karachristos, A. et al. Microsatellite instability and p53 mutations in hepatocellular carcinoma. Mol. Cell Biol. Res. Commun. 2, 155–161 (1999).
Kawai, H. et al. Quantitative evaluation of genomic instability as a possible predictor for development of hepatocellular carcinoma: comparison of loss of heterozygosity and replication error. Hepatology 31, 1246–1250 (2000).
Maggioni, M. et al. Molecular changes in hepatocellular dysplastic nodules on microdissected liver biopsies. Hepatology 32, 942–946 (2000).
Park, Y.M. et al. Microsatellite instability and mutations of E2F-4 in hepatocellular carcinoma from Korea. Hepatol. Res. 17, 102–111 (2000).
Piao, Z., Kim, H., Malkhosyan, S. & Park, C. Frequent chromosomal instability but no microsatellite instability in hepatocellular carcinomas. Int. J. Oncol. 17, 507–512 (2000).
Roncalli, M. et al. Fractional allelic loss in non-end-stage cirrhosis: correlations with hepatocellular carcinoma development during follow-up. Hepatology 31, 846–850 (2000).
Saeki, A. et al. Lack of frameshift mutations at coding mononucleotide repeats in hepatocellular carcinoma in Japanese patients. Cancer 88, 1025–1029 (2000).
Sun, M. et al. An early lesion in hepatic carcinogenesis: loss of heterozygosity in human cirrhotic livers and dysplastic nodules at the 1p36-p34 region. Hepatology 33, 1415–1424 (2001).
Chen, T.C. et al. p16INK4 gene mutation and allelic loss of chromosome 9p21-22 in Taiwanese hepatocellular carcinoma. Anticancer Res. 20, 1621–1626 (2000).
Okabe, H. et al. Comprehensive allelotype study of hepatocellular carcinoma: potential differences in pathways to hepatocellular carcinoma between hepatitis B virus-positive and -negative tumors. Hepatology 31, 1073–1079 (2000).
Chen, Y.J. et al. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology 119, 431–440 (2000).
Guan, X.Y. et al. Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer 29, 110–116 (2000).
Marchio, A. et al. Distinct chromosomal abnormality pattern in primary liver cancer of non-B, non-C patients. Oncogene 19, 3733–3738 (2000).
Tornillo, L. et al. Marked genetic similarities between hepatitis B virus-positive and hepatitis C virus-positive hepatocellular carcinomas. J. Pathol. 192, 307–312 (2000).
Wong, N. et al. Genomic aberrations in human hepatocellular carcinomas of differing etiologies. Clin. Cancer Res. 6, 4000–4009 (2000).
Zondervan, P.E. et al. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J. Pathol. 192, 207–215 (2000).
Balsara, B.R. et al. Human hepatocellular carcinoma is characterized by a highly consistent pattern of genomic imbalances, including frequent loss of 16q23.1-24.1. Genes Chromosomes Cancer 30, 245–253 (2001).
Rao, U.N. et al. Comparative genomic hybridization of hepatocellular carcinoma: correlation with fluorescence in situ hybridization in paraffin-embedded tissue. Mol. Diagn. 6, 27–37 (2001).
Wilkens, L. et al. Differentiation of liver cell adenomas from well-differentiated hepatocellular carcinomas by comparative genomic hybridization. J. Pathol. 193, 476–482 (2001).
Laes, J. et al. Alterations in P19ARF in rodent hepatoma cell lines but not in human primary liver cancer. Cancer Genet. Cytogenet. 117, 118–124 (2000).
Wong, N. et al. A comprehensive karyotype study of human hepatocellular carcinoma by spectral karyotyping. Hepatology 32, 1060–1068 (2000).
Strauss, B.S. Frameshift mutation, microsatellites, and mismatch repair. Mutat. Res. 437, 195–203 (1999).
Loeb, L.A. A mutator phenotype in cancer. Cancer Res. 61, 3230–3239 (2001).
Stewart, S.A. & Weinberg, R.A. Telomerase and human tumorigenesis. Semin. Cancer Biol. 10, 399–406 (2000).
Kane, J.M. III et al. Chronic hepatitis C virus infection in humans: induction of hepatic nitric oxide synthase and proposed mechanisms for carcinogenesis. J. Surg. Res. 69, 321–324 (1997).
Hussain, S.P. et al. Increased p53 mutation load in nontumorous human liver of Wilson disease and hemochromatosis: oxyradical overload diseases. Proc. Natl Acad. Sci. USA 97, 12770–12775 (2000).
Nishikawa, M. et al. Somatic mutation of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Res. 61, 1843–1845 (2001).
Yamamoto, H. et al. Infrequent widespread microsatellite instability in hepatocellular carcinoma and non-HCC tissues. Oncology Rep. 7, 725–729 (2000).
Shi, C.Y. et al. Codon 249 mutation of the p53 gene is a rare event in hepatocellular carcinomas from ethnic Chinese in Singapore. Br. J. Cancer 72, 146–149 (1995).
Terris, B. et al. Close correlation between β-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene 18, 6583–6588 (1999).
Hsu, H.C. et al. β-Catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am. J. Pathol. 157, 763–770 (2000).
Satoh, S. et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 24, 245–250 (2000).
Stern, M.C. et al. Hepatitis B, aflatoxin B1, and p53 codon 249 mutation in hepatocellular carcinomas from Guangxi, People's Republic of China, and a meta-analysis of existing studies. Cancer Epidemiol. Biomarkers Prev. 10, 617–625 (2001).
Mao, T.L., Chu, J.S., Jeng, Y.M., Lai, P.L. & Hsu, H.C. Expression of mutant nuclear βcatenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J. Pathol. 193, 95–101 (2001).
Wilkens, L., Bredt, M., Flemming, P., Klempnauer, J. & Heinrich Kreipe, H. Differentiation of multicentric origin from intra-organ metastatic spread of hepatocellular carcinomas by comparative genomic hybridization. J. Pathol. 192, 43–51 (2000).
van Oijen, M.G. & Slootweg, P.J. Oral field cancerization: carcinogen-induced independent events or micrometastastic deposits? Cancer Epidemiol. Biomarkers Prev. 9, 249–256 (2000).
Ng, I.O., Liang, Z.D., Cao, L. & Lee, T.K. DLC-1 is deleted in primary hepatocellular carcinoma and exerts inhibitory effects on the proliferation of hepatoma cell lines with deleted DLC-1. Cancer Res. 60, 6581–6584 (2000).
Emmert-Buck, M.R. et al. Laser capture microdissection. Science 274, 998–1001 (1996).
Tao, L.C. Oral contraceptive–associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer 68, 341–347 (1991).
Liotta, L. & Petricoin, E. Molecular profiling of human cancer. Nature Rev. Genet. 1, 48–56 (2000).
Hsu, H.C., Cheng, W. & Lai, P.L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 57, 5179–5184 (1997).
Lau, W.Y. et al. Differential gene expression of hepatocellular carcinoma using cDNA microarray analysis. Oncol. Res. 12, 59–69 (2000).
Honda, M., Kaneko, S., Kawai, H., Shirota, Y. & Kobayashi, K. Differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology 120, 955–966 (2001).
Lu, T. et al. Application of cDNA microarray to the study of arsenic-induced liver diseases in the population of Guizhou, China. Toxicol. Sci. 59, 185–192 (2001).
Okabe, H. et al. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 61, 2129–2137 (2001).
Shirota, Y., Kaneko, S., Honda, M., Kawai, H.F. & Kobayashi, K. Identification of differentially expressed genes in hepatocellular carcinoma with cDNA microarrays. Hepatology 33, 832–840 (2001).
Xu, L. et al. Expression profiling suggested a regulatory role of liver-enriched transcription factors in human hepatocellular carcinoma. Cancer Res. 61, 3176–3181 (2001).
Yamashita, T. et al. Serial analysis of gene expression in chronic hepatitis C and hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 282, 647–654 (2001).
Pollack, J.R. et al. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat. Genet. 23, 41–46 (1999).
Lindblad-Toh, K. et al. Loss of heterozygosity analysis of small-cell lung carcinomas using single-nucleotide polymorphism arrays. Nat. Biotechnol. 18, 1001–1005 (2000).
Risch, N.J. Searching for genetic determinants in the new millennium. Nature 405, 847–856 (2000).
Grisham, J.W. Interspecies comparison of liver carcinogenesis: implications for cancer risk assessment. Carcinogenesis 18, 59–81 (1997).
Kemp, C.J. Comparative hepatocellular cancer genetics. Am. J. Pathol. 154, 975–977 (1999).
Fausto, N. Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin. Liver Dis. 19, 243–252 (1999).
Thorgeirsson, S.S., Factor, V.M. & Snyderwine, E.G. Transgenic mouse models in carcinogenesis research and testing. Toxicol. Lett. 112–113, 553–555 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thorgeirsson, S., Grisham, J. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31, 339–346 (2002). https://doi.org/10.1038/ng0802-339
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0802-339
This article is cited by
-
BLVRA exerts its biological effects to induce malignant properties of hepatocellular carcinoma cells via Wnt/β-catenin pathway
Journal of Molecular Histology (2024)
-
Bio-diagnostic performances of microRNAs set related to DNA damage response pathway among hepatitis C virus-associated hepatocellular carcinoma patients
Journal of Genetic Engineering and Biotechnology (2023)
-
Potential role of human umbilical cord stem cells-derived exosomes as novel molecular inhibitors of hepatocellular carcinoma growth
Apoptosis (2023)
-
Custom gene expression panel for evaluation of potential molecular markers in hepatocellular carcinoma
BMC Medical Genomics (2022)
-
RBM47 inhibits hepatocellular carcinoma progression by targeting UPF1 as a DNA/RNA regulator
Cell Death Discovery (2022)